Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.57 suppl.1 San José Nov. 2009
Phenotypic and molecular variation in the green and black poison-dart frog Dendrobates auratus (Anura: Dendrobatidae) from Costa Rica
Lisa D. Patrick1 & Mahmood Sasa2, 3
1. Dept. of Biology, Texas Tech University, Lubbock, Texas, USA; lisa_d_patrick@yahoo.com
2. Instituto Clodomiro Picado, Universidad de Costa Rica, 11501-2060, San José, Costa Rica.
3. Organization for Tropical Studies (OTS), San José, Costa Rica; msasamarin@gmail.com
Abstract: The green and black poison-dart frog Dendrobates auratus exhibits high intraspecific variation in hue color and pattern throughout its range, making it a very popular species in the pet trade. We analyzed the correspondence between color variation and molecular variation of D. auratus from Costa Rica using RAPD analysis. Twenty-six random primers were analyzed for variation in 99 individuals from seven populations. Color pattern was scored from digital images of the dorsal and ventral views. In general, frogs from the Caribbean coast had significantly more light coloration than black color but cannot be grouped by population based only on hue pattern. Only 3 RAPD primers were found to be polymorphic, representing a total of 16 loci. Most of the molecular variation encountered here occurs within populations, thus making unclear the degree of population structure and differentiation. Further examination of COI mtDNA sequences from our samples also supports these results. Partial Mantel correlations suggested that the pattern of molecular variation is not congruent with the variation in color pattern in this species, an outcome that is discussed in terms of phenotypic evolution. Rev. Biol. Trop. 57 (Suppl. 1): 313-321. Epub 2009 November 30.
Key words: Dendrobates auratus, poison-dart frogs, RAPD, aposematism, color polymorphism, La Selva, Costa Rica.
The existence of color pattern polymorphism within and among species is a widespread phenomenon in Anura that has received considerable attention in recent years (Myers & Daly 1983, Heyer 1997, Hoffman & Blouin 2000). In general, physiological mechanisms underlying color variation involve hormonemediated changes in chromatophore projections, which subsequently induce temporal variation in color tones and pattern (Frost-Mason et al. 1994, Hoffman & Blouin 2000).
Less known is the ecological and evolutionary role of body coloration that usually is though to acquire different functions depending on the natural history context of the species. Color might be important in camouflaging the anuran within its surroundings, thus contributing to predator avoidance. Conversely, cryptic coloration might be beneficial to ambush and capture potential prey (Duellman & Trueb 1994). Other anurans exhibit brightly body coloration, a feature that characterizes many species in the families Dendrobatidae, Bufonidae, and Ranidae. In these families, bright coloration in toxic or distasteful species allows them to advertise their unpalatability to potential predators (Myers & Daly 1976, Duellman & Trueb 1994) or –in the case of mimetic species– help a non-toxic frog resemble a truly aposematic species (Myers & Daly 1983).
The frogs of the subfamily Dendrobatinae (Frost 2007) provide an exceptional model to study evolutionary factors that intervene in color polymorphism among populations. Fiftytwo species, commonly known as the poisondart frogs, are recognized in the subfamily, and are distributed from Isthmian Central America south to the Orinoco and Amazon Basins. These species are diurnal and display extensive polymorphisms in terms of hue color and patterns, features that also make them of great interest for commercial trade and captive breeders. One of the most dramatic examples is the strawberry poison frog, Oophaga pumilio, that exhibits more than 15 different color morphs throughout its distribution in Costa Rica and Panama (Summers et al. 2003, Hagemann & Pröhl 2007).
Extreme color variation has also been observed in the green poison-dart frog Dendrobates auratus (Girard 1854). This species is sympatric with O. pumilio in most of its distribution through the Caribbean lowlands from southern Nicaragua, to western Panama, but extends its range to northwestern Colombia (Savage 2002). Isolated populations of D. auratus also occur along the southwestern Pacific lowlands of Costa Rica and Panama. Frogs of this species usually have black and green blotches, but this pattern aries among populations: the dark blotches can be ebony, bronze, and even gold, whereas the green can be light, blue and cream (Savage 2002). Despite this dramatic variation, a truthful analysis of color variation is still pending for D. auratus throughout its range, and it is not clear if observed phenotypic variation matches genetic divergence among populations. As a first step to analyze color variation in D. auratus, here we evaluate the amount of color polymorphism among and within populations throughout Costa Rica. We analyze the level of molecular variation using random polymorphic DNA fragments (RAPD) markers and mitochondrial DNA sequences. Our goals were to determine if a correlation exists between molecular divergence and phenotypic divergence among populations, or if phenotypic variation in D. auratus can be attributed to environmental factors instead.
Materials and Methods
Sampled localities: In Costa Rica, D. auratus is a common inhabitant of lowland wet forests and forest edges of the Caribbean and south Pacific regions. In order to represent the extent of its distribution, during the dry season of 2002, we sampled seven localities along its range (coordinates; number of individuals): 1) Chilamate/Puerto Viejo de Sarapiqui (10°2718.83" N, 84°0422.04" W and 10° 2843.76" N, 84°0117.14" W; 22), 2) Cariari (10° 1223.56" N, 83° 4657.70" W; 22), 3) Guayacán de Siquirres (10° 0227.64" N, 83° 3246.41" W; 21), 4) Cahuita (9° 4400.63" N, 82° 4949.91" W; 6), 5) Jacó (9° 3530.45" N, 84° 3729.20" W; 14), 6) Quebrada Culebra (9° 1958.23" N, 83° 5658.94" W; 8), and 7) Bahía Ballena (9° 0856.24" N, 83° 4522.27" W; 6) for a total of 99 individuals. The first four localities occur along the Caribbean lowlands, whereas the last three are situated in the Pacific. Minimum distance between localities is 30 km, between Quebrada Culebra and Bahía Ballena. Caribbean and Pacific populations are geographically isolated from each other, as the average heights of the mountain range system that runs along central axis of the country is greater than (above 1000m) the altitudinal limit of the species in Costa Rica (~600 m, Savage, 2002). Except from Cariari where we sampled in primary forest, all frogs were collected from secondary growth forest in all other localities.
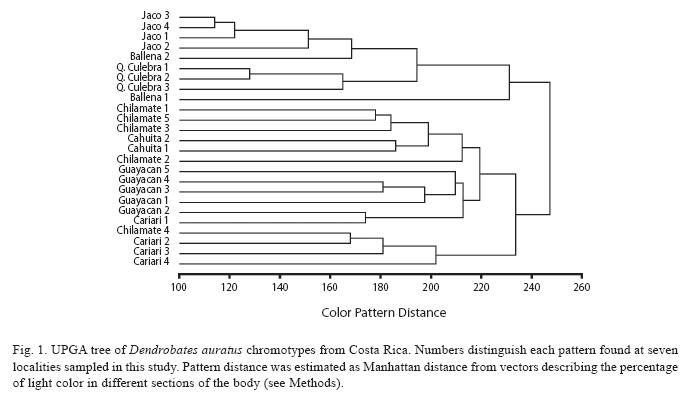
Color pattern: Both the dorsal and ventral side of each captured frog was photographed with a Nikon CoolPix 995 digital camera to examine color pattern. Frogs were marked and released soon after. Images were then analyzed using Adobe Photoshop® (version 6.0). Images were first divided into an eightysquare grid based on morphology. Then, each square was coded on a scale of 1 to 5 based on its light color content (1 = 100% green, 2 = 75%, 3 = 50%, 4 = 25%, 5 = 0%). The resulting codes for both dorsal and ventral images were grouped for each individual frog to create a vector of 160 elements. We calculated Manhattan distances among the created color vectors and grouped them using UPGA as the amalgamation rule using Statistica® (StatSoft, version 6.0).
DNA extraction: In the field, a toe from each frog was clipped and preserved in 70% ethanol until DNA extraction. It has been shown that toe-clips are reliable source of DNA and posses a minimum risk to the animal (Kimberling et al. 1996). To extract DNA, the toe samples were cut into small pieces and then mixed with 600 μl of sodium dodecyl sulfate (SDS) buffer. Next, 5.0 μL of Proteinase K was then added and samples were placed in a 37oC hot water bath for at least 4 hours. After digestion, total DNA was extracted using the PCI:CI method. DNA was quantified using a TD-700 Fluorometer (Turner Designs, California) and then stored at -20oC until needed.
RAPD amplification: Polymerase chain reactions (PCR) were used to amplify random polymorphic DNA fragments (RAPD) following the methods of Williams et al. (1990). Amplification was carried out in 25 microliter reactions containing 25 ng genomic DNA, 10X buffer (MgCl2 included, Finnzymes, Finland), 10mM dNTP mix (Applied Biosystems, USA), 1U Taq Polymerase (Finnzymes, Finland), and 5uM of the respective RAPD primer. Thermal cycling (Minicycler, MJ Research, Massachusetts, USA) consisted of 45 repetitions of 94oC for 1 minute, 35oC for 1 minute, and 72oC for 2 minutes. The 72oC step was held for 10 minutes in the final cycle. Amplified DNA was electrophoresed on 1% agarose gels and stained with ethidium bromide. We screened 26 primers (Operon Technologies, California, USA) across seven populations to identify markers that produced polymorphic bands. DNA extraction and amplification was conduced at OTSs La Selva Biological Station, Costa Rica.
DNA Sequencing: A 523 bp fragment of the cytochrome oxidase mitochondrial gene (CO1) was amplified following the protocols described in Lamar & Sasa (2003). Amplification was carried out in 25 microliter reactions containing 2.5μM of each primer (COIa and COIf, Palumbi et al. 1991). PCR products were cleaned using the DNA extraction kit (Fermentas ®). Sequencing was performed using BDT 3.0 Ready Reaction Cycle Sequencing Kit and a Perkin ElmerABI377 DNA automated sequencer. Electropherograms were analyzed for sequence ambiguities and mismatch, and the sequences aligned with a sequence of Oophaga pumilio (Genbank accession number: AF097500) using Sequecher.
Data analysis: For RAPD analysis, samples were scored using the presence/absence of each distinct band across all samples for the same primer. Genetic relationships within and among populations were evaluated by analysis of molecular variance (AMOVA) using Arlequin (version 2.0, Schneider et al. 2000.) Estimates of genetic distance (Nei 1978) between all pairs of frogs were obtained and relative distances analyzed using cluster analysis (UPGMA), Manly (1986). Phylogeographic analyses were conducted using PAUP* (Swofford version 4.0b1-b2; Sinauer Associates), under maximum likelihood.Because the methods for phylogenetic inference depend on their underlying sequence divergence model, 10 models were examined to find the one that best fits the data using ModelTest (version 2.1, Posada & Crandall 1998). The models are: Jukes-Cantor, Kimura 2 parameter, Tamura-Nei equal frequencies, Kimura 3 parameter, SYM, Felsenstein81, Hasegawa-Kishino-Yano, Tamura-Nei, Kimura 3 parameter unequeal base frequencies, and General time reversible (see references in Posada & Crandall 1998).
Simple and partial-Mantel tests (Manly 1986) were conducted to evaluate correlations of molecular divergence with geographic, environmental and color pattern distances. Environmental distances were constructed over estimations of annual maximum temperature, daily sun hours, mean monthly precipitation, and annual precipitation among all studied localities.
For the Mantel tests, the observed patterns of color variation are contrasted to several hypothetized factors simultaneously, in such a way that we can detect intercorrelation among the independent factors, and/or the additive effect among putative factors (Malhotra & Thorpe 2000). Matrix correlations were implemented in ZT (Bonnet & Van de Peer 2002). Since the matrix elements are not independent, standard parameter tests are not appropriate for these correlations. So, in ZT the probability of rejecting the null hypothesis of no association is estimated by comparing the correlation coefficient with its distribution obtained after randomizing rows and columns of one of the matrices.
Results
A total of 99 individuals D. auratus were observed, all showing a characteristic black and green hue coloration pattern. Across all frogs, blotch design and pattern could be classified into 24 distinct color patterns (=chromotypes). Interestingly, while diverse patterns were observed among all individuals, overall color patterns emerged when comparing frogs between coastal regions. More specifically, frogs in the Caribbean lowlands exhibited a higher overall percentage of light coloration in their bodies (48 to 52% green), than those inhabiting the Pacific lowlands (25 to 35% green).
Distinct patterns were also observed among populations, but only in the Pacific region at Jaco and Quebrada Culebra sites. Other populations in the Pacific and most populations in the Caribbean region exhibited great variation among individuals, as inferred from the high values of the color distances observed (Fig. 1). This population-level variation resulted in great overlap among chromotypes and made it difficult to distinguish the locality of origin for specimens collected within each population solely using color (Fig. 1).
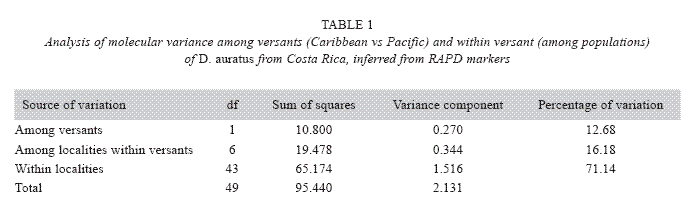
From a total of 26 RAPD markers examined here, only three resulted polymorphic: primers AD3 (TCTCGCCTAC), AD16 (AACGGGCGTC), and AD19 (CTTGGCACGA). Collectively, these primers scored 16 loci. Except for three dominant alleles found in samples from either the Caribbean versant (a segment of 2000 bp) or Pacific (one segment 300 bp, another of 2500 bp), no unique markers were found at any of the studied localities. Furthermore, the analysis of molecular variance (Table 1) reveals that most of the observed variation occurs among individuals within populations, and that the divergences among individuals at different versants are minor. Overall, these results indicate that no genetic structure of D. auratus populations in Costa Rica can be inferred using RAPD markers.
On the other hand, low molecular divergence in Costa Rican D. auratus was also supported by sequences of the mitochondrial CO1 gene. A total of 14 haplotype sequences were found in our sample (Fig. 2), but the mean pairwise divergence among haplotypes ranged from 0.00 to 0.08 substitutions per site. A phylogeographic analysis based on these sequences (Fig. 2) reveals that D. auratus samples form a monophyletic group, but the pattern of relationships among populations remains unclear, as the observed molecular divergence was extremely low.
The molecular distances among individuals inferred from CO1 sequences are slightly related to their spatial distances (r = 0.44, P < 0.042), an expected result if we consider the geographic scale among our samples. Moreover, a slight but significant correlation was found between color pattern distances and molecular distances (r = 0.46, P = 0.036), but the association disappears when geographic distance is taken into account (partial Mantel r = 0.33, P = 0.126).
Environmental variables (maximum temperature, daily sun hours, mean monthly precipitation, and annual precipitation) did not show any correlation with the patterns of coloration (r < 0.036, P > 0.24 in all cases).
Discussion
In Costa Rica, Dendrobates auratus exhibit moderate color pattern variation, but molecular variation, estimated with RAPD analyses, did not support the patterns of divergence observed in color types. Lack of correspondence between molecular variation and color polymorphism has been reported in other species of dendrobatids.
In Oophaga pumilio, Summers et al. (1997) (see also Hagemann & Pröhl 2007) did not find a correlation between the extended color variation and the levels of mitochondrial DNA divergence relative to other dendrobatid species that show little color divergence in the same region.
They suggested that neutral divergence in allopatry is unlikely to have caused the geographical differences observed among Panamanian populations of O. pumilio. Conversely, sexual selection related with female parental care is proposed as the causal evolutionary mechanism that drives divergence in coloration among populations in this species (Summers et al. 1997, 2003). Under this view, a Fisherian runaway model might explain color diversity associated with the different populations in D. pumilio: since females invest more in parental care, female choice for male ornamentation will be strong. As a result, females "can afford" to select males with different color types in different populations. In this model, sexual dimorphism may be reduced by genetic correlations between sexes or by convergence thru selection by predators (Summers et al. 2003).
This model thus will fit better in cases whereparental care is mostly driven by females, but will not be expected to cause variation in cases with strong male investment in offspring since selection of new "types" might be restricted as the cost of choice is more expensive for females. Dendrobates auratus is a polygynic species in which both sexes have high investment in their offspring (Summers 1990). Female D. auratus are territorial and fight other females to prevent them from mating with their males whereas parental care (moisture and care of eggs, carrying of tadpoles) is mainly conducted by the male (Summers 1989, 1990, Summers et al. 1999, Savage 2002). Thus, the argument of sexual selection as the main force driving color pattern divergence in D. auratus is unsoundly, as variation in male traits (including coloration) should be limited for females advantage.
The inability of the molecular analyses to support differences observed in color patterns among populations may also be due to our method of genetic discrimination. RAPD analyses are widely used to account for molecular divergence because of their low cost, effectiveness, and easy implementation in the lab (Williams et al. 1990). However, the RAPD technique assumes that only dominant markers are expressed in the gels, and that these alleles are inherited following Mendelian genetics. Since the recessive alleles do not migrate to the same position on the gel, the level of genetic diversity can be underestimated in RAPD methodology (Pérez et al. 1998). Furthermore, the levels of molecular variation inferred from RAPD markers in amphibians are usually low, as Kimberling et al. (1996) suggested after finding 8.5% polymorphic markers among populations of Lithobates (=Rana) pipiens. While this constraint implies that prudence should guide inferences about population structure and molecular divergence based on RAPD analyses (Pérez et al. 1998), we believe that the levels of molecular variation reported here truly reflect low levels of genetic divergence in D. auratus from Costa Rica, as is supported by the mitochondrial gene (CO1) sequences. Interestingly, observed divergences among haplotypes of this gene were similar to those encountered at the intraspecific level in other dendrobatid populations (Summer et al. 1997).
An alternative hypothesis to explain the low levels of genetic divergence observed among populations is that recent introductions of D. auratus have occurred in sampled localities, thereby increasing the genetic diversity of a specific population. This situation could also lead to high chromotype overlap observed within populations. As D. auratus is one of the most attractive frogs along its distribution, it plays a large role in attracting tourists. Thus, multiple introductions of D. auratus to various localities throughout the country might be more frequent than previously thought due to increased tourism in Costa Rica in recent times. For example, in Chilamate, Donnelly & Guyer (2005) reported that individuals of D. auratus were introduced by a local employee of a tourist lodge in 1986. Nowadays, a population of D. auratus has been reported to be established in this region. The presence of an introduced population of D. auratus in Chilamate is further supported by the lack of records of D. auratus observed in the area before late 1980s, as well as the recent appearance of D. auratus at La Selva Biological Station in nearby Puerto Viejo de Sarapiqui. As La Selva is one of the most extensively herpetologically studied sites in the Tropics, this is good evidence that the popular green and black frog is a relative newcomer to the area. Unfortunately, the lack of unique markers within a single population prevents us from explicitly testing this hypothesis.
Nevertheless, results from this study do show that while the majority of frogs from Chilamate group together by chromotype, there are two groups of frogs from Chilamate that are more closely associated colormetrically with frogs from Cariari and Cahuita (Fig. 1).
Additionally in this study, while we observed distinct color pattern differences between Pacific and Carribean coasts, we did not find evidence for association between environmental factors and color variation among Costa Rican populations.
Likewise, no evidence has been found that other dendrobatids undergo color variation in response to abiotic factors (Summers et al., 2003). These results are surprising when compared to the great number of ecogenetic adaptations that result from different environmental conditions that have been documented for a broad arrangement of organisms (Thorpe 1996, Malhotra & Thorpe 2000, Manier 2004).
Despite these results, diversity in color traits observed within populations of dart frogs might still be a consequence of environmentally mediated changes in the past in combination with intrinsic plasticity in color traits (i.e. phenotypic plasticity). Natural selection might target phenotypic plasticity allowing the genome to absorb the changes (Dudley & Schmidt 1996). Non-genetic effects (those environmentally induced on phenotypes) could shift the range of phenotypes expressed and in this way alter the strength and direction of selection acting in genes frequencies (West-Eberhard 1989). Under this view, adaptative phenotypes can originate rapidly in a population with little genetic change by means of correlated shifts in the expression of plastic traits, making genes "followers and not leaders of phenotypic evolution" (West-Eberhard 1989; 2003). This process could explain the diversity of chromotypes observed in populations of dendrobatids, and the low genetic divergence among them reported here and in other studies (Summers et al. 1997, 2003). Also, it might explain why the Bocas del Toro populations of D. auratus and O. pumilio are so variable in hue pattern, with less variation encounter within Costa Rican populations of both species.
Our results confirm the existence of two distinct color phases on each side of the mountain axis of the country (as noticed by Savage 2002), but neither RAPD nor our analysis of color pattern, nor color correlations with environmental variables provide a means of matching an individual to a specific population. In effect, this prevents the use of these methodologies in identifying the population of origin for individuals of D. auratus in Costa Rica, an urgent need in light of frog seizures by government officials. To be able to appropriately release these and other captured dendrobatids back to their populations of origin in an effort response to abiotic factors (Summers et al., 2003). These results are surprising when compared to the great number of ecogenetic adaptations that result from different environmental conditions that have been documented for a broad arrangement of organisms (Thorpe 1996, Malhotra & Thorpe 2000, Manier 2004). Despite these results, diversity in color traits observed within populations of dart frogs might still be a consequence of environmentally mediated changes in the past in combination with intrinsic plasticity in color traits (i.e. phenotypic plasticity). Natural selection might target phenotypic plasticity allowing the genome to absorb the changes (Dudley & Schmidt 1996). Non-genetic effects (those environmentally induced on phenotypes) could shift the range of phenotypes expressed and in this way alter the strength and direction of selection acting in genes frequencies (West-Eberhard 1989). Under this view, adaptative phenotypes can originate rapidly in a population with little genetic change by means of correlated shifts in the expression of plastic traits, making genes "followers and not leaders of phenotypic evolution" (West-Eberhard 1989; 2003). This process could explain the diversity of chromotypes observed in populations of dendrobatids, and the low genetic divergence among them reported here and in other studies (Summers et al. 1997, 2003). Also, it might explain why the Bocas del Toro populations of D. auratus and O. pumilio are so variable in hue pattern, with less variation encounter within Costa Rican populations of both species
Our results confirm the existence of two distinct color phases on each side of the mountain axis of the country (as noticed by Savage 2002), but neither RAPD nor our analysis of color pattern, nor color correlations with environmental variables provide a means of matching an individual to a specific population. In effect, this prevents the use of these methodologies in identifying the population of origin for individuals of D. auratus in Costa Rica, an urgent need in light of frog seizures by government officials. To be able to appropriately release these and other captured dendrobatids back to their populations of origin in an effort to conserve genetic diversity, more sophisticated color analyses or more suitable genetic markers should be developed.
Acknowledgments
Randall Valverde greatly contributed to this project by assisting with frog collection, photographs, and interviews. Laboratory work would not have been possible without the help and support of Sergio Vargas, Viviana Arce, Gabriela Azofeifa and Marietta Flores at the Instituto Clodomiro Picado (UCR). The authors acknowledge Federico Bolaños and William Lamar for reviewing and improving the manuscript.
This project was supported by a U.S. Fulbright Fellowship to LDP, and by Vicerrectoría de Investigación, Universidad de Costa Rica (Project 741-A1-021).
Resumen
La rana venenosa Dendrobates auratus posee una gran variación intraespecífica en tonos y patrones de coloración a lo largo de toda su distribución, lo que la hace una especie muy reconocible entre las especies de dendrobátidos. Analizamos la correspondencia entre variación de coloración y variación molecular de D. auratus de Costa Rica empleando análisis de RAPDs. La variación resultante en veintiséis "primers" aleatorios fue analizada en 93 individuos de siete localidades en Costa Rica. El patrón de coloración fue evaluado de imágenes digitales del dorso y vientre para los mismos individuos. En general, las ranas provenientes de localidades en la costa Caribe tienen significativamente una coloración más clara, con menos proporción de color negro que las de localidades en la vertiente Pacífica, pero no pueden ser agrupadas por localidad basadas simplemente en el patrón de coloración. Solamente tres RAPD "primers" fueron encontrados polimórficos, representando un total de 16 loci. Mucha de la variación molecular encontrada habita dentro de poblaciones, lo que hace difícil determinar el grado de estructura poblacional y diferenciación. La reexaminación posterior de secuencias del gen mitocondrial CO1 también apoya estos resultados. Correlaciones parciales de matrices (test de Mantel) sugieren que el patrón de variación molecular no es congruente con la variación en el patrón de coloración en esta especie, un resultado que es discutido en términos de evolución fenotípica.
Palabras clave: Dendrobates auratus, rana venenosa, RAPD, aposematismo, polimorfismo, policromatismo, La Selva, Costa Rica.
Received 23-II-2009. Corrected 01-VII-2009. Accepted 08-XI-2009.
Referentes
Bonnet, E. & Y. Van de Peer. 2002. ZT: A software tool for simple and partial Mantel tests. J. Stat. Software 7: 1-12. [ Links ]
Dudley, S.A. & J. Schmidt. 1996. Testing the adaptative plasticity hypothesis: Density dependent selection on manipulated stem length in Impatiens capensis. Amer. Nat. 147: 445-465. [ Links ]
Duellman, W.E. & L. Trueb. 1994. Biology of Amphibians. 2nd edition. Johns Hopkins University Press, Baltimore, Maryland. [ Links ]
Frost-Mason, S., Morrison, R. & K. Mason. 1994. Pigmentation. Amphibian Biology (ed. H. Heatwole). Surrey Beatty & Sonns, Norton, New South Wales. [ Links ]
Guyer, C. & M.A. Donnelly. 2005. Amphibians and Reptiles of La Selva, Costa Rica, and the Caribbean Slope: A Comprehensive Guide. University of California Press, California. [ Links ]
Hagemann, S. & H. Pröhl. 2007. Mitochondrial paraphyly in a polymorphic poison frog species (Dendrobatidae: D. pumilio). Mol. Phyl. Evol. 45(2): 740-747. [ Links ]
Heyer, W.R. 1997. Geographic variation in the frog genus Vanzolinius (Anura: Leptodactylidae). Proc. Biol. Soc. Washington 110: 338-365. [ Links ]
Hoffman, E.A., & M.S. Blouin. 2000. A review of colour and pattern polymorphism in anurans. Biological J. Linn. Soc. 70: 633-665. [ Links ]
Kimberling, D.N., A.R. Ferreira, S.M. Shuster & P. Keim. 1996. RAPD marker estimation of genetic structure among isolated northern leopard frog populations in the south-western USA. Mol. Ecol. 5: 521-529. [ Links ]
Lamar, W.W. & M. Sasa. 2003. A new species of hognose pitviper genus Porthidium from the southwestern Pacific of Costa Rica (Serpentes: Viperidae). Rev. Biol. Trop. 51(3-4):797-804. [ Links ]
Malhotra, A. & R.S. Thorpe. 2000. The dynamics of natural selection and vicariance in the Dominican anole: Patterns of within island molecular and morphological divergence. Evolution 54:245-258. [ Links ]
Manier, M.K. 2004. Geographic variation in the long-nosed snake, Rhinocheilus lecontei (Colubridae): Beyond the subspecies debate. Biol. J. Linn. Soc. 83: 65-85. [ Links ]
Manly, B.F.J. 1986. Multivariate Statistical Methods: A primer. Chapman and Hall, London. [ Links ]
Myers, C.W. & J.W. Daly. 1976. Preliminary evaluation of skin toxins and vocalizations in taxonomic and evolutionary studies of poison dart frogs (Dendrobatidae). Bull. Amer. Mus. Nat. Hist. 157: 177-262. [ Links ]
Myers, C.W. & Daly J.W. 1983. Dart-poison frogs. Sci. Amer. 248: 120-133. [ Links ]
Nei, M. 1978. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89: 583-590. [ Links ]
Palumbi, S.R., A. Martin, S. Romano, W.O. Mcmillan, L. Stice & G. Grabowski. 1991. A Simple Fools Guide to PCR. University of Hawaii, Honolulu, Hawai. [ Links ]
Perez, T., J. Albornoz, and A. Dominguez. 1998. An evaluation of RAPD fragment reproducibility and nature. Mol. Ecol. 7: 1347-1357. [ Links ]
Posada, D. & K.A. Crandall. 1998. MODELTEST: Testing the models of DNA substitution. Bioinformatics 14: 817-818. [ Links ]
Schneider, S., D. Roessli & L. Excoffier. 2000. Arlequin: A software for population genetic data analysis. Genetics and Biometry Lab, Department of Anthropology, University of Geneva, Italy. [ Links ]
Savage, J.M. 2002. The Amphibians and Reptiles of Costa Rica: A Herpetofauna between two Continents, between two Seas. The University of Chicago Press. Chicago. 934 pp. [ Links ]
Summers, K. 1989. Sexual selection and intra-female competition in the green poison-dart frog Dendrobates auratus. Anim. Behav. 37: 797-805. [ Links ]
Summers, K. 1990. Parental care and the cost of polygyny in the green poison-dart frog. Behav. Ecol. Sociobiol. 27: 307-313. [ Links ]
Summers, K., E. Bermingham, L. Weigt, S. McCafferty & L. Dahlstrom. 1997. Phenotypic and genetic divergence in three species of dart-poison frogs with contrasting parental behavior. J. Heredity 88: 8-13. [ Links ]
Summers, K., T.W. Cronin & T. Kennedy. 2003. Variation in spectral reflectance among populations of Dendrobates pumilio, the strawberry poison frog, in the Bocas del Toro Archipielago, Panama. J. Biogeography 30: 35-53. [ Links ]
Summers, K., L.A. Weigt, P. Boag, and E. Bermingham. 1999. The evolution of female parental care in poison frogs of the genus Dendrobates: Evidence from mitochondrial DNA sequences. Herpetologica 55:254-270. [ Links ]
Thorpe, R.S. 1996. The use of DNA divergence to help determine the correlates of evolution of morphological characters. Evolution 50: 524-531. [ Links ]
West-Eberhard, M.J. 1989. Phenotypic plasticity and the origins of diversity. Ann. Rev. Ecol. Syst. 20: 249-278. [ Links ]
West-Eberhard, M.J. 2003. Developmental Plasticity and Evolution. Oxford University Press, New York. Williams, J.G.K., A.R. Kubelik, K.J. Livak, J.A. Rafalski, & S.V. Tingey. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acid. Res. 18:6531-6535. [ Links ]
Internet Reference
Frost, Darrel R. 2007. Amphibian Species of the World: an Online Reference. Version 5.0 (1 February, 2007). Electronic Database accessible at http://research. amnh.org/herpetology/amphibia/index.php. American Museum of Natural History, New York, USA. [ Links ]