Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.57 suppl.1 San José Nov. 2009
Histology of somatic embryogenesis in rice (Oryza sativa cv. 5272)
Rafael Vega1, 4, Nelly Vásquez2, Ana M. Espinoza3, Andrés M. Gatica1 & Marta Valdez-Melara1
1. Escuela de Biología, Universidad de Costa Rica, 11501-2060, San Pedro, Costa Rica.
2. Centro Tropical de Investigación y Enseñanza (CATIE), Turrialba, Costa Rica.
3. Centro de Investigación en Biología Celular y Molecular (CIBCM), Universidad de Costa Rica, 11501-2060, San Pedro, Costa Rica.
4. Dirección actual: Fundación para la Innovación Tecnológica Agropecuaria, El Salvador.
Abstract: Rice (Oryza sativa cv. 5272) embryogenic calli were obtained from mature zygotic embryos culture on Murashige & Skoog (1962) medium supplemented with 2.5 mg/l 2,4- dichlorophenoxyacetic acid. Histological analysis of somatic embryogenesis revealed that after two weeks of culture of explants on the callus induction medium, somatic embryo development began with a cluster of proembryogenic cells in the peripheral region of the calli. The outer cell layer of embryogenic calli consisted of small and isodiametric cells with a dense cytoplasm and a prominent nucleus and nucleolus; whereas the inner cell layer is composed of large cells with small nucleus and large vacuole. These embryogenic cells underwent a series of organized divisions and formed the proembryo with a well-defined protodermis. Rev. Biol. Trop. 57 (Suppl. 1): 141-150. Epub 2009 November 30.
Key words: Oryza sativa, rice, in vitro culture, histology, somatic embryogenesis, morphogenesis, Costa Rica.
The totipotent character of plant cells allow that any differentiated cells that retains its nucleus has the ability to regenerate an entire new plant by organogenesis or somatic embryogenesis (SE) (Reynolds 1997, Fortes & Pais 2000). SE is the developmental process by which bipolar structures that resemble zygotic embryos are developed from haploid or diploid somatic cell through an orderly embryological stage without gametes fusion (Jiménez 2001, Jiménez 2005, Quiroz-Figueroa et al. 2006, Namasivayam 2007). Two types of somatic species embryogenesis are recognized: direct somatic embryogenesis (DSE) and indirect somatic impor embryogenesis (ISE). DSE is characterized by the induction of somatic embryos directly from pro-embryogenic cells from leaves, stem, microspores or protoplasts without the proliferation of calli, whereas in ISE somatic embryos are developed from friable embryogenic calli (Jiménez 2001, Molina et al. 2002, Quiroz-Figueroa et al. 2002b, Quiroz-Figueroa et al. 2006). Somatic embryogenesis is a unique process in plants and it is of remarkable interest for biotechnological applications such as clonal propagation, artificial seeds and genetic engineering (Quiroz-Figueroa et al. 2006, Namasivayam 2007). Precisely, when somatic embryogenesis is integrated with conventional breeding programs and molecular and cell biological techniques, it provides a valuable tool to enhanced genetic improvement of crop (Quiroz-Figueroa et al. 2006).
As rice (Oryza sativa) is the most tant staple crop for one third of the world population, there is considerable interest in the development of new cultivars tolerant to biotic and abiotic stresses (Valdez et al. 1996b). Plant biotechnology represents an alternative to conventional breeding programs; nevertheless, integration of biotechnology into rice improvement through genetic engineering or mutagenesis requires a reliable and efficient in vitro culture system.
In rice, somatic embryogenesis is the most common regeneration pathway and has been obtained from roots, leaf bases of young seedlings, mature embryos, immature embryos, caryopses, microscopes, cell suspension, protoplast and young inflorescences (Kawata & Ishihara 1968, Inoue & Maeda 1980, Wernicke et al. 1981, Heyser et al. 1983, Abe & Futsuhara 1984, Raghavan Ram & Nabors 1984, Abe & Futsuhara 1985, Chen et al. 1985, Abe & Futsuhara 1986, Kavi Kishor & Reddy 1986, Raina et al. 1987, Hartke & Lorz, 1989, Koetje et al. 1989, Chowdhry et al. 1993, Valdez et al., 1996a,b, Gairi & Rashid 2004, Hoque & Mansfield 2004, Meneses et al. 2005, Ge et al. 2006). Some author present organogenesis and embryogenesis, occurring simultaneously, as the regeneration pathway (Boissot et al. 1990, Gairi & Rashid 2004).
Nevertheless, successful application of genetic engineering or mutagenesis techniques cannot be achieved if the processes leading to morphogenesis are not well understood (Fortes & Pais 2000). Therefore, the aim of this work was to describe the events leading to the development of plantlets from embryogenic calli obtained from Costa Rican rice "CR-5272" through histological analysis.
Materials and methods
Plant material and embryo isolation: Seeds of the commercial Costa Rican cultivar CR-5272 were surface-sterilized following the procedure described by Valdez et al. (1996). Briefly, the seeds were immersed in an aqueous 30% (v/v) Domestos (Lever Ltd. Warrington, G.B.) solution for 35 min and then rinsed five times in sterile distilled water. Disinfected seeds were soaked overnight in sterile distilled water to facilitate embryo isolation without damaging the scutellum. Embryos were excised under a binocular microscope using fine scalpels and forceps.
Callus induction: Ten disinfected seeds were cultured in each Petri dish (100x15 mm) containing 40 ml of callus induction medium (CIM) which consisted of MS mineral salts (Murashige & Skoog 1962) supplemented with 100 mg/l myo-inositol, 50 mg/l tryptophan, 2.5mg/l 2,4-dichlorophenoxyacetic acid (2,4D), 30 g/l sucrose and 3 g/l Phytagel. The pH was adjusted to 5.8 before autoclaving for 21 min at 121°C and 1.07 kg cm-2. Petri dishes were sealed with polyethylene food wrap (Glad, Costa Rica) and the explants were cultured in the dark at 26±2 °C.
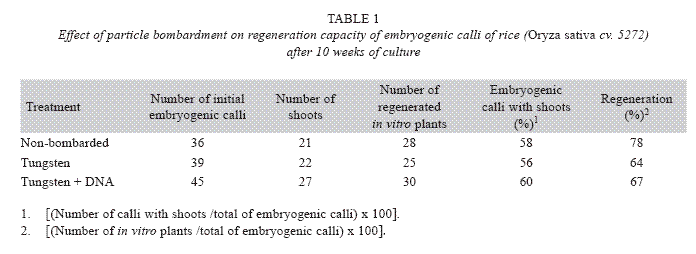
In order to investigate the effect of particle gun bombardment on the regeneration capacity, embryogenic calli were bombarded with tungsten particles coated with DNA or tungsten particles only using 80 psi and a Particle Inflow Gun (Finer et al. 1992). Additionally, non-bombarded embryogenic calli were included as controls. The number of shoots and in vitro plants per embryogenic calli were determined after ten weeks of culture on regeneration medium that consisted of MS mineral salts supplemented 0.5 mg/l 6- benzylaminopurine (BAP), 0.05 mg/l naphthaleneacetic acid (NAA), 30 g/l sucrose and 3 g/l Phytagel. The explants were cultured in the dark at 26±2 °C.
The percentage of embryogenic calli with shoots [(Number of calli with shoots /total of embryogenic calli)x100] and the percentage of regeneration [(Number of in vitro plants /total of embryogenic calli)x100] were calculated.
Histological study: Histological analysis of the embryogenic calli and zygotic embryos were performed according to Boissot et al. (1990). Embryogenic calli were collected 5, 10, 15 and 20 days after cultured on callus induction medium. Both types of tissues were fixed in FAA (formalin-acetic acid-ethanol) for 24 h. Then, the samples were dehydrated in a graded series of ethanol (70, 95 and 100%) for 1 h each one and embedded in paraffin wax. Samples were cut into 9-12 µm sections and were stained with PAS-Hematoxylin, PAS-aniline blue black or eosine-alcyan blue. Macroscopic features were photographed using a stereoscope and the photographs of the histological study were taken using an OLYMPUS IX51 inverted microscope.
Results
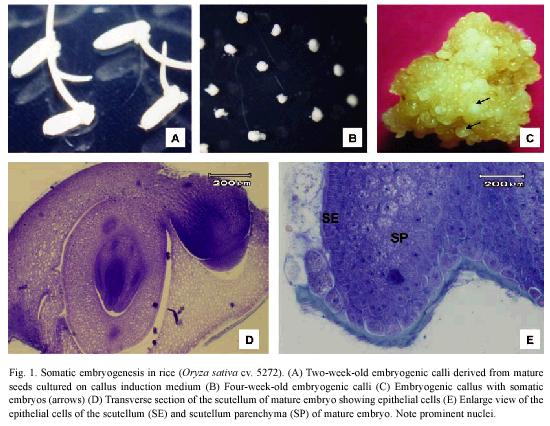
Mature embryos produced friable yellowish calli (Fig. 1A-C) derived from the scutellum (Fig. 1D) after two weeks of culture on callus induction medium. The epithelial cells of the scutellum are columnar with a dense cytoplasm and a prominent nucleus and nucleolus (Fig. 1D). Three days after zygotic embryos were culture on the CIM, transverse section showed that embryogenic regions were formed from the more mitotically active epithelial cells of the scutellum (Fig. 1E).
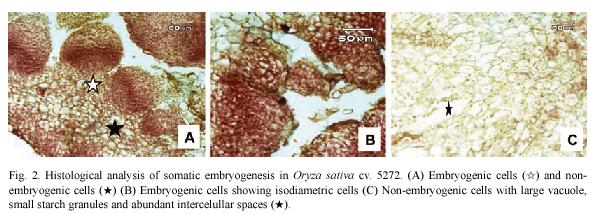
Histological observations on embryogenic calli revealed two types of cells (Fig. 2A). The outer cell layer consisted of 9-12 rows of small and isodiametric cells with a dense cytoplasm and a prominent nucleus and nucleolus (Fig. 2B). The initial divisions of these cells were periclinal and anticlinal. These observations denote an active metabolism and indicate that external calli cells resembled meristematic cells. In contrast, in the inner cell layer, large cells with small nucleus and large vacuole were observed (Fig. 2C). Between these two cell layers, there is a layer of 3 rows composed of laterally compressed cells with green stained primary cell walls. The center of the embryogenic calli consisted of large cells with small nucleus and starch granules and abundant intercellular spaces (Fig. 2C).
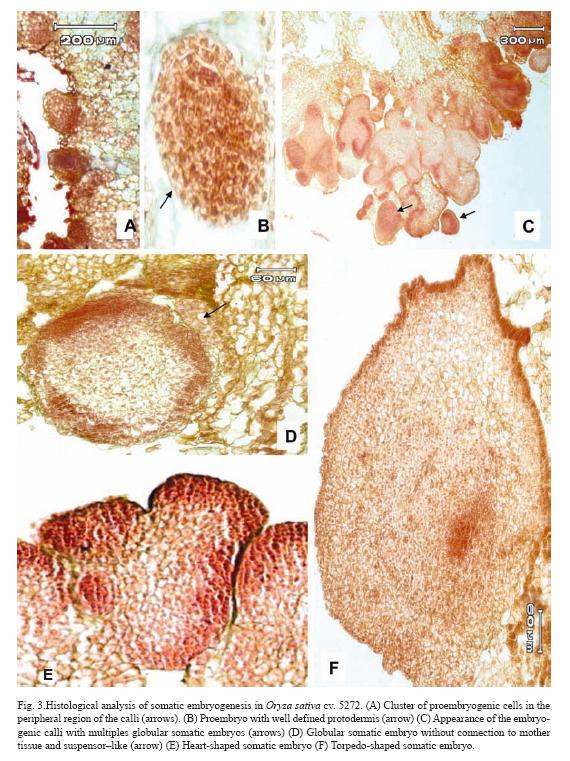
After 10 days of culture, cluster of proembryogenic cells in the peripheral region of the calli were observed (Fig. 3A). It was observed that immediately after the proembryogenic region the cells increased in size with respect to the formers indicating different stages of differentiation (Fig. 3A). The next step in SE is the formation of the proembryo (Fig. 3B) which showed a well-defined protodermis (Fig. 3B). At 20 days after initiation of culture, these pro-embryos continued a series of organized division and gave rise to globular somatic embryos which are delimited by a well defined layer of epidermal cells with conspicuous nucleus (Fig. 3D). These somatic embryos had no apparent vascular connection with the mother tissue and had a suspensor–like (Fig. 3D). After ten days of culture on regeneration medium, globular somatic embryos developed into heart-shape somatic embryos, which consisted of cells with prominent nucleus and dense cytoplasm (Fig. 3E). After 15 days of culture on regeneration medium torpedo-shaped somatic embryos were observed. These somatic embryos showed signs of polarization with apical and radical meristems in opposite poles (Fig. 3F).
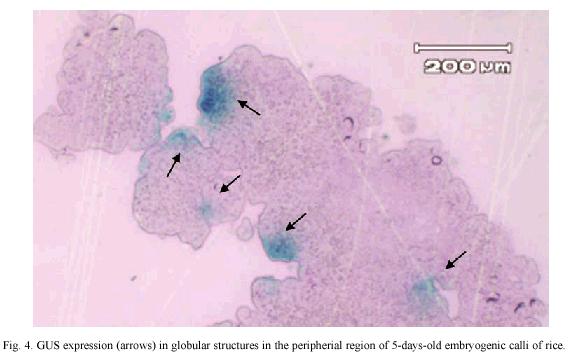
GUS expression was determined on rice embryogenic calli after 24 h of particle bombardment. The presence of abundant and prominent foci in the calli periphery were observed, which indicated that tungsten particles coated with the DNA has been delivery into the cell and cause no damage to the embryogenic cells (Fig. 4). The number of shoots, regenerated in vitro plants, percentage of embryogenic calli with shoots and the percentage of regeneration was similar among non-bombarded calli and the calli bombarded with tungsten or tungsten plus DNA (Table 1).
Discussion
In the application of genetic engineering or mutagenesis techniques in rice improvement through in vitro culture, knowledge on the morphogenetic pathway and location of the precise origin of competent cells is important (Mendoza et al. 1993). This study presents histological aspects of callus initiation and somatic embryo formation on epithelial cells of the scutellum of mature zygotic embryo of rice (Oryza sativa cv. 5272). Our results confirmed previous observations in wheat immature embryos (Ozias-Akins & Vasil 1983), maize immature embryos (Kamo et al. 1985), immature and mature zygotic embryos of Pannicum maximum Jacq. (Lu & Vasil 1985) and rice (Hartke & Lorz 1989, Jones & Rost 1989, Koetje et al. 1989, Biswas et al. 1994, Rueb et al. 1994, Valdez et al. 1996).
Maeda & Radi (1991) emphasized that the main function of the epithelial cells of the rice scutellum is the absorption of sugars and plant growth regulators, which could explain their high metabolic activity and the facility to dedifferentiate and gave rise to embryogenic calli in comparison with the rest of cells of the scutellum. Jones & Rost (1989) indicated that the scutellum epithelium is a modified layer of columnar cells at the interfase of the embryo with the endosperm and it is an active tissue during germination and has been reported as the site of amylase synthesis in grasses.
The morphological and anatomical observations indicate that somatic embryos may arise from one cell or a group of cells (Quiroz-Figueroa et al. 2002b, Quiroz-Figueroa et al. 2006). When somatic embryos have unicellular origin, coordinated cell divisions are observed and the embryos are connected to the maternal tissue by a suspensor-like. In contrast, multicellular origin is characterized by no coordinated cell divisions and somatic embryos are observed as a protuberance and fused to the maternal tissue (Quiroz-Figueroa et al. 2006). Our histological observations showed that somatic embryos originated from the more external cells of the embryogenic calli, agreeing with previous observations in sugarcane (Jane-Ho & Vasil 1983), Guinea Grass (Lu & Vasil 1985) and oil palm (Schwendiman et al. 1988).
During somatic embryogenesis induction in the rice cultivar 5272 two distinguishable clusters, embryogenic and non-embryogenic cells, were observed. In the embryogenic systems described until now in maize (Fransz & Schel 1991a,b), sugarcane (Falco et al. 1996), pearl millet (Taylor & Vasil 1996), cork oak (Puigderrajols et al. 2001), sugar beet (Moghaddam & Taha 2005) and coffee (Quiroz-Figueroa et al. 2002a, Gatica et al. 2007a) the embryogenic cells show characteristics common to meristematic cells: high division rates, cells are isodiametric and small with a dense cytoplasm with several starch grains, large nucleus and prominent nucleolus, small vacuoles, thin cell walls and a higher metabolic activity (Quiroz-Figueroa et al. 2006). Moreover, the main morphological characteristic of somatic embryos is the bipolarity and the absence of connection with the explant vascular tissue (Falco et al. 1996, Gatica et al. 2007a). The cells containing starch grains observed in rice somatic embryogenesis have been related to the acquisition of embryogenic potencial and indicates the high nutritional requirement of cell populations during the process (Fransz & Schel 1991b, Apezzato-Da-Gloria & Machado 2004).
The histological observations of the rice embryogenic calli at different developmental stages showed an internal organization of the calli. Thus, the external layers consisted of meristematic cells, which gave rise to clusters of embryogenic cells and somatic embryos. Whereas, the interior of the callus consisted of parenchymatic cells with a less visible nucleus and many vacuoles. Some of these parenchymatic cells are broken and gave rise to the internal space of the callus. These observations confirmed the study of Fransz & Schel (1991a) in maize that indicate that the formation of prominent air spaces is due to the differentiation and ultimate death of vacuolated cells in the cell aggregates.
Since most somatic cells are not naturally embryogenic, an induction phase is required for the cells to acquire embryogenic competence (Namasivayam 2007). In DSE it has been suggested that embryogenic cells are present and simple require favorable conditions for embryo development while in ISE requires the re-determination of differentiated cells (Quiroz-Figueroa et al. 2002b). Jiménez (2001) clearly distinguishes between embryogenic and competent cells. The former ones are those cells that have completed their transition from a somatic state to one in which no further application of exogenous stimuli are necessary to produce somatic embryo. On the other hand, the term competent cells is restricted to that cells that have reached the transitional state and have started to become embryogenic but still require exogenous stimuli application. In this sense, some of the epithelial cells of the scutellum in presence of 2,4-D acquired an competent state and gave rise to the embryogenic calli alter 5 days of culture. It has been showed that the auxin 2,4-D plays an important role in the dedifferentiation and cell division in rice somatic embryogenesis (Meneses et al. 2005).
On the other hand, optimization of physical, biological and environmental parameters for particle bombardment is necessary for transient or stable gene expression in any plant tissue (Tee & Maziah 2005). Moreover, size of tissue is an important factor that needs to be considered for multiplication and regeneration of bombarded explants. Since particle bombardment involves the penetration of heavy metal particles into intact cells or tissues, microparticles hit may provoke various levels of tissue wounding and damage that can hind plant regeneration (Tadesse et al. 2003). Our histological observations suggest that biolistic cause no damage to the embryogenic calli and therefore these technique could be used for genetic transformation as reported in rice (Li et al. 1993), maize (Valdez et al. 2004) and coffee (Rosillo et al. 2003, Ribas et al. 2005, Gatica et al. 2007b).
In conclusion, our results provide further information on the morphology and development of friable callus in rice (Oryza sativa cv. 5272) tissue culture.
Acknowledgments
The authors are grateful to reviewers for the helpful discussion, critical review and English correction of the manuscript. Financial support was provided by the Vicerrectoría de Investigación of the University of Costa Rica (Project No. 111-96-000) and the Rockefeller Foundation.
Resumen
Los estudios anatómicos e histológicos de los callos embriogénicos de arroz (Oryza sativa) mostraron que estos se originan del epitelio escutelar de los embriones cigóticos maduros. Al crecer en un medio Murashige y Skoog (1962) suplementado con 2.5 mg/l 2,4-D, presentan grupos de células embriogénicas en las zonas periféricas, las cuales a su vez darán lugar a la formación de proembriones y de embriones somáticos. Las células de los callos embriogénicos se caracterizan por tener núcleo y nucleolo conspícuos, forma isodiámetrica, citoplasma denso y están acompañadas de células adyacentes con abundantes gránulos de almidón. Los embriones somáticos completan su desarrollo para dar formación a plántulas completas, al estar en un medio de regeneración. Los estudios histológicos permitieron observar la expresión transitoria del gen uidA en grupos de células de las capas más externas de los callos embriogénicos sometidos al método de transformación genética por biobalística.
Palabras clave: Oryza sativa, arroz, cultivo in vitro, embriogénesis somática, histología, morfogénesis, Costa Rica.
Received 11-X-2007. Corrected 29-VIII-2009. Accepted 04-X-2009.
References
Abe, T. & Y. Futsuhara. 1984. Varietal difference of plant regeneration from root callus tissues in rice. Jap. J. Breed. 34: 147-155. [ Links ]
Abe, T. & Y. Futsuhara. 1985. Efficient plant regeneration by somatic embryogenesis from root callus tissue of rice (Oryza sativa L.). J. Plant Physiol. 121: 111-118. [ Links ]
Abe, T. & Y. Futsuhara. 1986. Genotypic variability for callus formation and plant regeneration in rice (Oryza sativa L.). Theor. Appl. Genet. 72: 3-10. [ Links ]
Apezzato-Da-Gloria, B. & S. Machado. 2004. Ultrastructural analysis of in vitro direct and indirect organogenesis. R. Bras. Bot. 27: 429-437. [ Links ]
Biswas, G.C., P.K. Burkhardt, J. Wunn, A. Kloti & Y. Potrykus. 1994. Fertile indica rice plant regenerated from protoplast isolated from scutellar tissue of inmature embryos. Plant Cell Rep. 13: 528-532. [ Links ]
Boissot, N., M. Valdez & E. Guiderdoni. 1990. Plant regeneration from leaf and seed-derived calli and suspension cultures of the African perennial wild rice, Oryza longistaminata. Plant Cell Rep. 9: 447-450. [ Links ]
Chen, T.H., L. Lam & S.C. Chen. 1985. Somatic embryogenesis and plant regeneration from cultured young inflorescences of Oryza sativa L. (rice). Plant Cell Tiss. Org. Cult. 4: 51-54. [ Links ]
Chowdhry, C.N., A.K. Tyagi, N. Maheshwari & S.C. Maheshwari. 1993. Effect of L-proline and L-tryptophan on somatic embryogenesis and plantlet regeneration of rice (Oryza sativa L. cv. Pusa 169). Plant Cell Tiss. Org. Cult. 32: 357-361. [ Links ]
Falco, M.C., B.M. Januzzi Mendez, A. Tulmann Neto & B. Appezzato Da Gloria. 1996. Histological characterization of in vitro regeneration of Saccharum sp. R. Bras. Fisiol. Veg. 8: 93-97. [ Links ]
Finer, J.J., P. Vain, M.W. Jones & M. D. Mcmulen. 1992. Development of the particle inflow gun for DNA delivery to plant cell. Plant Cell Rep. 11: 323-328. [ Links ]
Fortes, A.M. & M.S. Pais. 2000. Organogenesis from internode-derived nodules of Humulus lupulus L. var Nugget (Cannabinaceae): histological studies and changes in the starch content. Am. J. Bot. 87: 971-979. [ Links ]
Fransz, P.F. & J.H.N. Schel. 1991a. Cytodifferentiation during the development of friable embryogenic callus of maize (Zea mays). Can. J. Bot. 69: 26-33. [ Links ]
Fransz, P.F. & J.H.N. Schel. 1991b. An ultrastructural study on the early development of Zea mays somatic embryos. Can. J. Bot. 69: 858-865. [ Links ]
Gairi, A. & A. Rashid. 2004. TDZ-induced somatic embryogenesis in non-responsive caryopses of rice using a shot treatment with 2,4-D. Plant Cell Tiss. Org. Cult. 76: 29-33. [ Links ]
Gatica-Arias, A.M., G. Arrieta & A.M Espinoza. 2007. Comparison of three in vitro protocols for direct somatic embryogenesis and plant regeneration of Coffea arabica L. cvs. Caturra and Catuaí. Agronomía Costarricense. 31: 85-94. [ Links ]
Ge, X., Chu Z., Y. Lin & S. Wang. 2006. A tissue culture system for different germoplasms of indica rice. Plant Cell Rep. 25: 392-402. [ Links ]
Hartke, S. & H. Lörz. 1989. Somatic embryogenesis and plant regeneration from various indica rice (Oryza sativa L.) genotypes. J. Genet. Breed. 43: 205-214. [ Links ]
Heyser, J.W., T.A. Dykes, K.J. Demott, M.W. Nabors. 1983. High frecuency, long term regeneration of rice from callus culture. Plant Science Lett. 29: 175-182. [ Links ]
Hoque, Md. & J.W. Mansfield. 2004. Effect of genotype and explant age on callus induction and subsequent regeneration from root-derived callus of Indica rice genotypes. Plant Cell Tiss. Org. Cult. 78: 217-233. [ Links ]
Inoue, M. & E. Maeda. 1980. Thiamine as a factor of organ formation in rice callus culture. Japan J. Crop Sci. 49: 1-7. [ Links ]
Jane-Ho W. & I.K. Vasil. 1983. Somatic embryogenesis in sugarcane (Saccharum officinarum L.): Growth and plant regeneration from embryogenic cell suspension cultures. Ann. Bot. 51: 719-726. [ Links ]
Jiménez, V. 2001. Regulation of in vitro somatic embryogenesis with emphasis on the role of endogenous hormones. R. Bras. Fisiol. Veg. 13:196-223. [ Links ]
Jiménez, V. 2005. Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regul. 47:91-110. [ Links ]
Jones, T.J. & T.L. Rost. 1989. The developmental anatomy and ultraestructural of somatic embryos from rice (Oryza sativa L.) scutellum ephitelial cells. Bot. Gaz. 150: 41-49. [ Links ]
Kamo, K.K., M.R. Becwar & T.K. Hodges. 1985. Regeneration of Zea mays L. from embryogenic callus. Bot. Gaz. 146: 327-334. [ Links ]
Kavi, Kishor, P.B.K. & G.M. Reddy 1986. Regeneration of plants from long-term cultures of Oryza sativa L. Plant Cell Rep. 5: 391-393. [ Links ]
Kawata, S.I. & A. Ishihara. 1968. The regeneration of rice plant, Oryza sativa L., in the callus derived from the seminal root. Proc. Japan. Acad. 44: 549-553. [ Links ]
Koetje, D. S., H. D. Grimes, Y.C. Wang, T.K. Hodges. 1989. Regeneration of Indica rice (Oryza sativa L.) from primary callus derived from immature embryos. J. Plant Physiol. 35: 184-190. [ Links ]
Li, L.Q., A. Kochko, C. Fauquet & R. Benchy. 1993. And improvement rice transformation system using the biolistic method. Plant Cell Rep. 12: 250-255. [ Links ]
Lu, C. & I.K. Vasil. 1985. Histology of somatic embryogenesis in Panicum maximun (Guinea Grass). 72: 1908-1913. [ Links ]
Maeda E. & S.H. Radi. 1991. Ultrastructural aspects of rice scutellum as related to seminal root cultures. Biotechnology in Agriculture and Forestry (Rice). 14: 78-91. [ Links ]
Mendoza, A.B., K. Hattori, T. Nishimura & Y. Futsuhara. 1993. Histological and sccaning electron microscopic observations on plant regeneration in mugbean cotyledon (Vigna radiata (L.) Wilczek) cultured in vitro. Plant Cell Tiss. Org. Cult. 32: 137-143. [ Links ]
Meneses, A., D. Flores, M. Muñoz, G. Arrieta & A.M. Espinoza. 2005. Effect of 2,4-D, hydric stress and light on indica rice (Oryza sativa) somatic embryogenesis. Rev. Biol. Trop. 53: 361-368. [ Links ]
Moghaddam, B.E. & R.M. Taha. 2005. Cellular behavior in embryogenic and no-embryogenic sugar beet calluses. In Vitro Dev. Biol.-Plant. 41: 465-469. [ Links ]
Molina, D., M. Aponte, H. Cortina & G. Moreno. 2002. The effect of genotype and explant age on somatic embryogenesis of coffee. Plant Cell Tiss. Org. Cult. 71: 117-123. [ Links ]
Murashige, T. & F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15:473-497. [ Links ]
Namasivayam, P. 2007. Acquisition of embryogenic competence during somatic embryogenesis. Plant Cell Tiss. Org. Cult. 90: 1-8. [ Links ]
Ozias-Akins, P. & I.K. Vasil. 1985. Nutrition of plant tissue cultures. In Cell culture and somatic cell genetics of plants. Edited by Indra K. Vasil. Academic Press, Inc. Vol. 2. Cap.4. pp 129-142. [ Links ]
Puigderrajols, P., G. Mir & M. Molinas. 2001. Ultrastructure of early secondary embryogenesis by multicellular and unicellular pathways in cork oak (Quercus suber L.). Amer. J. Bot. 87:179–189. [ Links ]
Quiroz-Figueroa, F.R., C.F.J. Fuentes-Cerda, R. Rojas-Herrera, V.M. Loyola-Vargas. 2002b. Histological studies on the developmental stages and differentiation of two different somatic embryogenesis systems of Coffea arabica. Plant Cell Rep. 20:1141-1149. [ Links ]
Quiroz-Figueroa, F.R., M. Méndéz-Zeel, F. Sánchez-TeyerR. Rojas-Herrera, V.M. Loyola-Vargas. 2002a. Differential gene expression in embryogenic and non-embryogenic clusters from cell suspensions cultures of Coffea arabica. J. Plant. Physiol. 159: 1-4. [ Links ]
Quiroz-Figueroa, F.R., R. Rojas-Herrera, R.M. Galaz-Avalos & V.M. Loyola-Vargas 2006. Embryo production through somatic embriogénesis can be used to study cell differentiation in plants. Plant Cell Tiss. Org. Cult. 86: 285-301. [ Links ]
Raghavan, Ram, N.V. & M.W. Nabors. 1984. Cytokinin mediated long-term, high-frequency plant regeneration in rice tissue cultures. Z. Pflanzenphysiol. 103: 315-323. [ Links ]
Raina, S.K., P. Sathish & K.S. Sarma. 1987. Plant regeneration from in vitro cultures of anthers and mature seeds of rice (Oryza sativa L) cv. Basmati-370. Plant Cell Rep. 6: 43-45. [ Links ]
Reynolds, T. 1997. Pollen embryogenesis. Plant Mol. Biol. 33: 1-10. [ Links ]
Ribas, A.F., A.K. Kobayashi, L.F.P. Pereira & L.G.E. Vieira. 2005. Genetic transformation of Coffea canephora by particle bombardment. Biol. Plant. 49: 493-497. [ Links ]
Rosillo, G., J. Acuña, A. Gaitan, & De M. Peña. 2003. Optimized DNA delivery into Coffea arabica suspension culture cells by particle bombardment. Plant Cell Tiss. Org. Cult. 74: 45-49. [ Links ]
Rueb, S., M. Leneman, R.A. Schilperoot & A.M. Hengsgens. 1994. Efficient plant regeneration throught somatic embryogenesis from callus induced on mature rice embryos (Oryza sativa L.). Plant Cell Tiss. Org. Cult. 36: 259-264. [ Links ]
Schwendiman, J., C. Pannetier & N. Michaux-Ferriere. 1988. Histology of somatic embryogenesis from leaf explants of the oil palm Elaeis guineensis. Ann. Bot. 62: 43-52. [ Links ]
Tadesse, Y., L. Sági, R. Swennen & M. Jacobs. 2003. Optimisation of transformation conditions and production of transgenic sorghum (Sorghum bicolor) via microparticle bombardment. Plant Cell Tiss. Org. Cult. 75: 1-18. [ Links ]
Taylor, M.G. & I.K. Vasil. 1996. The ultrastructure of somatic embryo development in pearl millet (Pennisetum glaucum; Poaceae). Amer. J. Bot. 83:28–44. [ Links ]
Tee, Ch.S. & M. Maziah. 2005. Optimization of biolistic bombardment parameters for Dendrobium Sonia 17 calluses using GFP and GUS as the reporter system. Plant Cell Tiss. Org. Cult. 80: 77-89. [ Links ]
Valdez, M., G. Garro & A.M. Espinoza. 1996a. Establishment of morphogenetic rice cell suspension cultures (Oryza sativa L.) in Costa Rica. Rev. Biol. Trop. 44-45: 593-595. [ Links ]
Valdez, M., K. Madriz & P. Ramírez. 2004. Un método de transformación genetica de maíz para conferirle resistencia ulterior a enfermedades virales. Rev. Biol. Trop. 52: 787-793. [ Links ]
Valdez, M., M. Muñoz, J.R. Vega & A.M. Espinoza. 1996b. Plant regeneration of indica rice (Oryza sativa L.) cultivars from mature embryo-derived calli. Rev. Biol. Trop. 44-45: 13-21. [ Links ]
Wernicke, W., R. Brettell, T. Wakiuika & I. Potrykus. 1981. Adventitious embryod and root formation from rice leaves. Z. Pflanzenphysiol. 103: 361-365. [ Links ]