Revista de Biología Tropical
versión On-line ISSN 0034-7744versión impresa ISSN 0034-7744
Rev. biol. trop vol.57 no.4 San José dic. 2009
Zooplankton assemblage of Oyun Reservoir, Offa, Nigeria
Moshood K. Mustapha
Department of Zoology, University of Ilorin, Ilorin, Nigeria; moonstapha@yahoo.com
Abstract: The influence of physico-chemical properties of Oyun Reservoir, Offa, Nigeria (a shallow tropical African reservoir) on its zooplankton composition and abundance were investigated at three stations for two years between January 2002 and December 2003. Diversity is not high: only three groups of zooplankton were found: Rotifera with eight genera; and Cladocera and Copepoda with three genera each. Rotifera dominated numerically (71.02%), followed by Cladocera (16.45%) and Copepoda (12.53%). The zooplankton was more prevalent during the rainy season, and there were variations in the composition and abundance along the reservoir continuum. Factors such as temperature, nutrients, food availability, shape and hydrodynamics of the reservoir, as well as reproductive strategies of the organisms, strongly influence the generic composition and population density of zooplankton. Prevention of ecological deterioration of the water body would greatly should result in a more productive water body, rich in zooplankton and with better fisheries. Rev. Biol. Trop. 57 (4): 1027-1047. Epub 2009 December 01.
Key words: zooplankton, Rotifera, Cladocera, Copepoda, reservoir, physico-chemical factors.
Reservoirs are considered favourable environments to the development of zooplankton communities, which may establish diversed assemblages in relatively short periods of time after impoundment (Rocha et al. 1999). Several factors usually contribute to the establishment of zooplankton communities in a reservoir, among which are good water quality, presence of nutrients, physico-chemical factors of water, availability of phytoplankton, hydrological characteristics of the reservoirs and reservoir ageing.
Once established, zooplankton assemblage usually influences energy flow through classical food chain, nutrient cycling and community population dynamics within the reservoir ecosystem. This ecological niche has also made them key actors in their top down grazing effect (trophic cascade) on the bottom up forces which plays pivotal roles in biomanipulation for lake restoration purposes (Carpenter & Kitchel 1993). The species composition, distribution, diversity and relative abundance of zooplankton of a reservoir could have significant impact on fisheries and public health of the reservoir and its users. Typical zooplankton assemblage of reservoirs is commonly constituted by Protozoa, Rotifera, Copepoda and Cladocera (Rocha et al. 1999). This assemblage often differ in diversity and abundance from reservoir to reservoir, from location to location within each reservoir, from geographical region to region and also with time (intra-annual and between years) and are structured by fish predation, competition, aquatic macrophytes (Jackson & Schmitz 1987) and physical, chemical and biological factors (Sampaio et al. 2002).
The objective of this paper is to investigate the species composition and relative abundance of zooplankton of Oyun Reservoir in relation to physico-chemical factors of the reservoir. This is with a view to understanding the contribution of the zooplankton community to the reservoir productivity, and managing the zooplankton population in their natural settings for sustainable fisheries.
Materials and methods
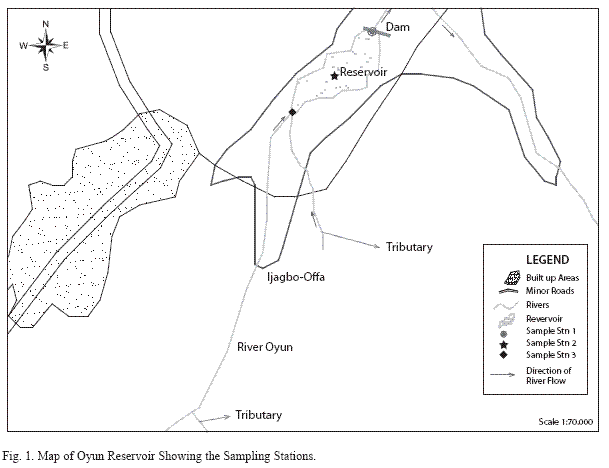
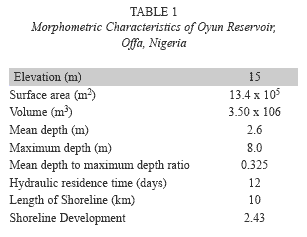
Study site: Oyun Reservoir (Fig. 1) is a tropical man-made reservoir created in Offa, Nigeria (8º30’N and 8º15’E), it was created by damming the perennial Oyun River in 1964, but greatly expanded in 1995 and with another expansion being proposed. The reservoir was constructed primarily to supply domestic water to the people of Offa and environs. It also supplies water to industries located within the area and provide water for general municipal uses. Subsistence fishing activities is carried out on the reservoir. The reservoir is eutrophic (Mustapha 2008) with diverse species of littoral plant occupying the shoreline length. The morphometric characteristics of the reservoir are listed in Table 1.
Physico-chemical sampling: Physicochemical characteristics of the water body were sampled monthly from three stations ranging in depth between 0-8m in Station 1, 0-6m in Station 2 and 0-5m in Station 3. Sampling was done between January 2002 and December 2003. Triplicate surface water samples were collected in 1-litre plastic water bottles and analyzed for dissolved oxygen, chemical oxygen demand, nitrate, phosphate, calcium, magnesium, total hardness, carbon dioxide, total alkalinity, sulphate and silica according to the standard methods for the examination of water and waste water (APHA 1998) and Hach (2003) procedures. Analyses requiring photometric measurement were determined using Hach spectrophotometer model DR/2500 according to Hach (2003) procedures. Temperature, pH, conductivity and total dissolved solids were measured in-situ using Hanna portable combo waterproof pH/EC/TDS/Temperature Tester model HI 98130. Transparency was evaluated using a standard 20cm diameter Secchi disc having black and white quarters.
Zooplankton sampling: Zooplankton samples were collected using a 25cm diameter zooplanktons net of 76μm much size monthly between January 2002 and December 2003. The net was towed vertically over a distance of 2m on the water surface due to the shallowness of the reservoir and the sample was collected into 50ml bottle and preserved in 10% formalin. A 5ml subsample was taken for identification under the microscope after the preserved sample has been homogenized. Identification was done only to generic level using keys compiled by Edmonson (1959), Whitford & Schumacher (1973), Jeje & Fernando (1986). Numerical estimations of the zooplankton were done using the drop method of Margalef (1976). The relative abundance of the various taxa was then calculated.
Statistical analyses of the results were done using the GLM procedure of statistical analysis system 9.1.3 (SAS Institute 2003). Monthly mean differences in stations, seasons (dry season, November-April and rainy season, May-October) and years were assessed by LSD using two-way ANOVA with test of significance at P<0.05. Multivariate analysis of co-variance using principal component analysis of physico- chemical variables and factorial correspondence analysis of the zooplancton abundance was used to investigate the correlation and level of dependence between the total zooplankton and the zooplankton groups with the physico- chemical factors. The test variables were stations, seasons and years. Statistical differences were considered at P< 0.05
Results
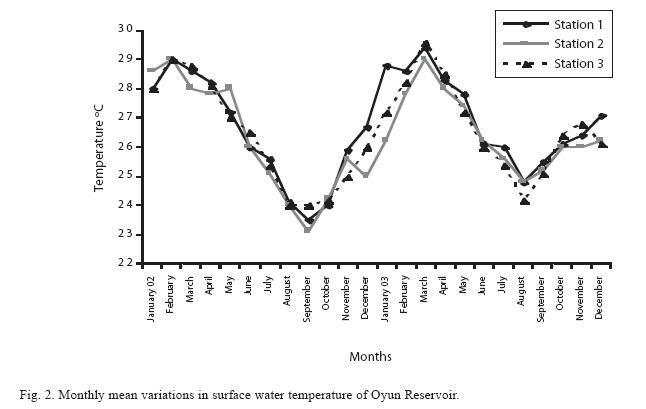
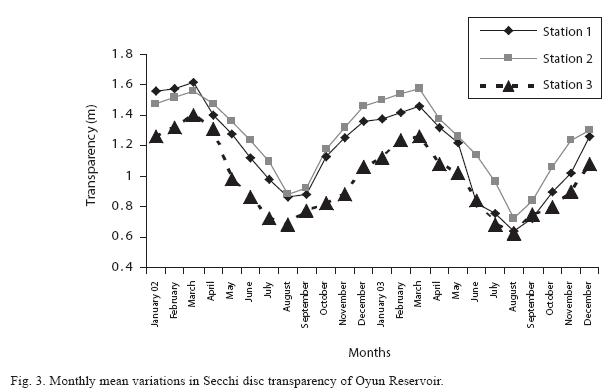
Physico-chemical factors: The mean monthly variation in the surface water temperature of the three stations is presented in Fig. 2. The temperature ranged between the lowest value of 23.1±0.5ºC obtained from Station 2 in September and the highest of 29.6±0.1ºC obtained from station 3 in March, 2003. Dry season temperature was significantly higher (P<0.05) than the wet season. No significant difference was seen among the stations and in the two years. Secchi disc transparency was the highest at station 1 with a mean value of 1.62±0.32m obtained in March 2002. Station 3 recorded the least Secchi disc transparency value with a mean of 0.62±0.8m obtained in August, 2003 (Fig. 3). The dry season, station 2 and year 2002 had significant higher transparency (P<0.05).
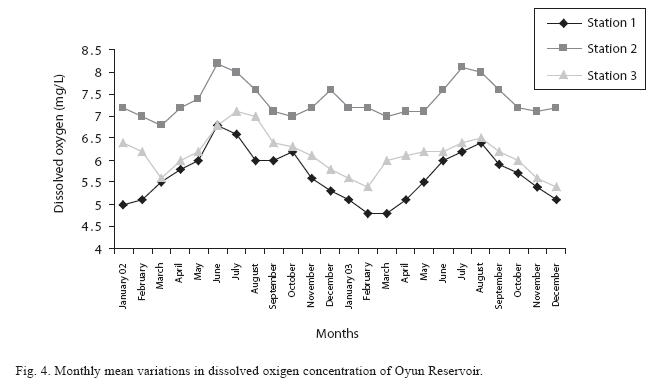
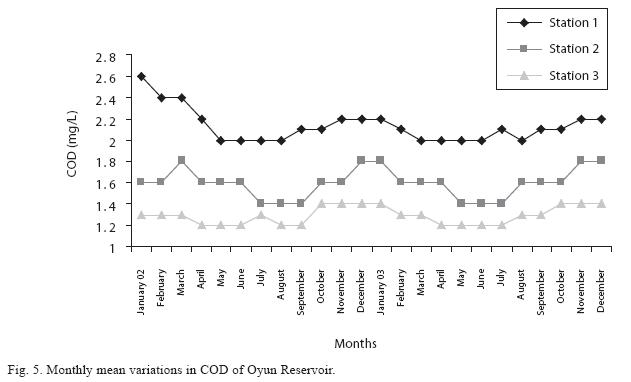
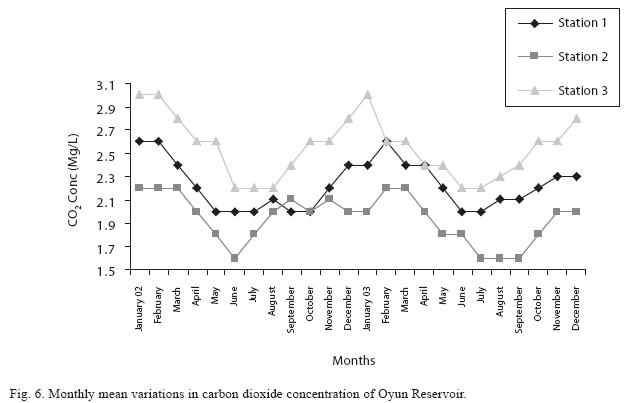
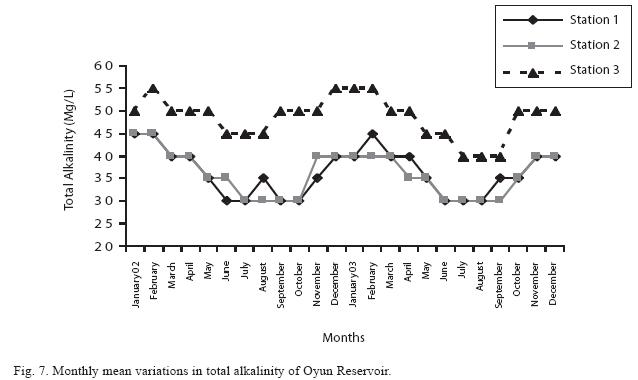
Dissolved oxygen fluctuated between lowest monthly mean of 4.8±0.25mg/L obtained in February and March 2003 from Station 1 and the highest monthly mean of 8.2±0.31mg/L recorded in June 2002 from Station 2 (Fig. 4). Statistical difference at P<0.05 was noticed in the dissolved oxygen concentration among the stations, (with station 2 having the highest concentration). The wet season values were significantly higher than dry season and year 2002 had a higher concentration than 2003. Chemical Oxygen Demand (COD) varied between 1.2±0.1mg/L and 2.6±0.2mg/L. COD was significantly higher in the dry season with Station 1 recording the highest concentration and station 3 recording the lowest concentration in the wet season (Fig. 5). There was no statistical difference in COD between the two years of the study. Carbon dioxide and total alkalinity showed similar pattern in their concentration among the stations and in the seasons. The two factors were statistically higher in the dry season as well as in Station 3. Carbon dioxide ranged between monthly mean of 1.6±0.2mg/L to 3.0±0.6mg/L (Fig. 6) while total alkalinity fluctuated between monthly mean of 30±2.6mg/L and 55±3.4mg/L (Fig. 7).
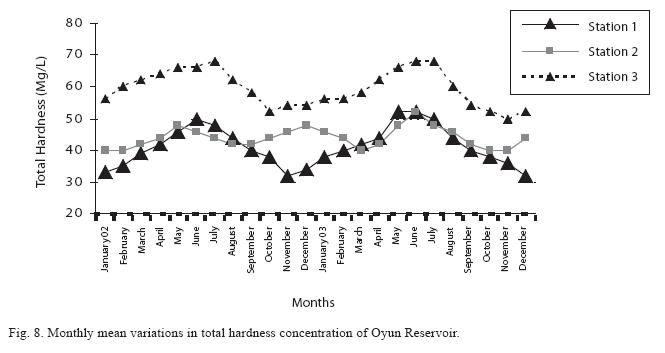
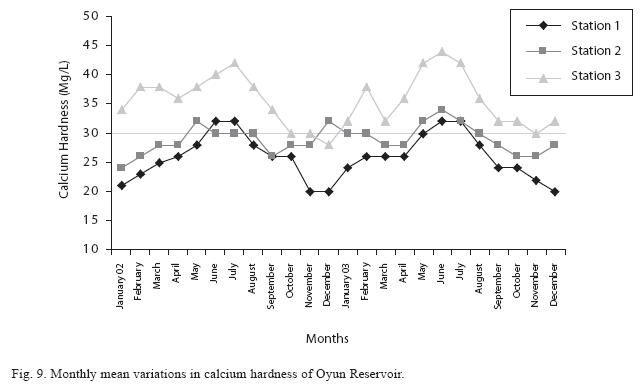
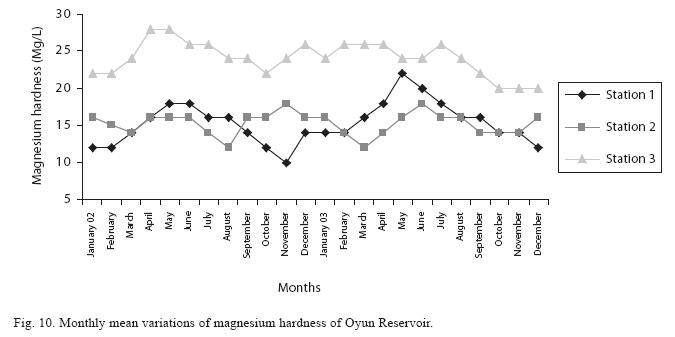
The total hardness value in the reservoir which is the sum of calcium and magnesium hardness concentrations was found to be significantly higher in the wet season. This was the same for calcium and magnesium ions. Station 3 showed significantly higher concentration of total, calcium and magnesium hardness than the other stations. The mean monthly range of the total hardness (32±0.5mg/L– 68±1.4mg/L), calcium hardness (20±0.1mg/L–44±1.8mg/L) and magnesium hardness (10±0.4mg/L– 28±0.6mg/L) are presented in Figures 8, 9 and 10 respectively. There was no significant difference in the concentration of these ions among the two years.
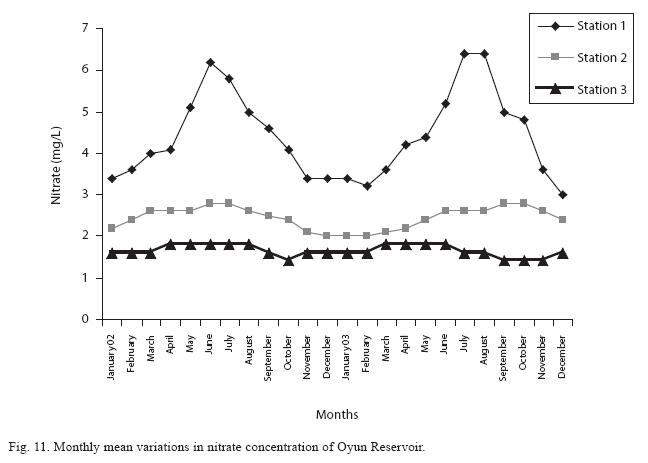
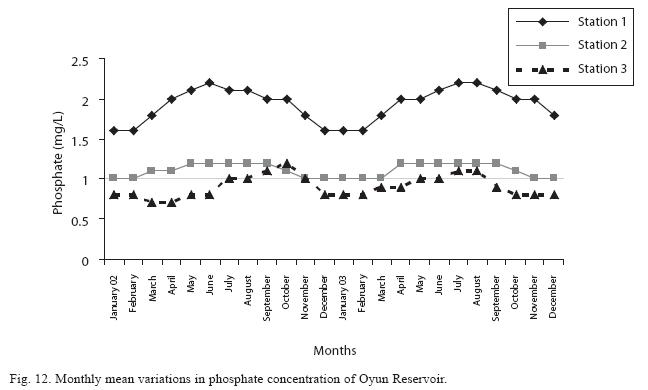
The highest monthly mean concentration of nitrate recorded was 6.4±0.3mg/L obtained from Station 1 at the peak of the rains in August 2003. A decrease was observed in the dry season with the lowest concentration of 1.4±0.1mg/L recorded from Station 3 in October 2003 (Fig. 11). ANOVA at P<0.05 shows significant difference in the nitrate concentration during the seasons and within the stations. Nitrate was higher in the raining season and the order of magnitude in the concentration among the stations was station 1> 2>3. Phosphate had the least concentration among the nutrients. It ranged between 0.7±0.0mg/L to 2.2±0.2mg/L (Fig. 12). Like nitrate, phosphate concentration was significantly higher in raining season and in station 1 (P<0.05). No significant difference occurred between the years of study in nitrate and phosphate concentrations.
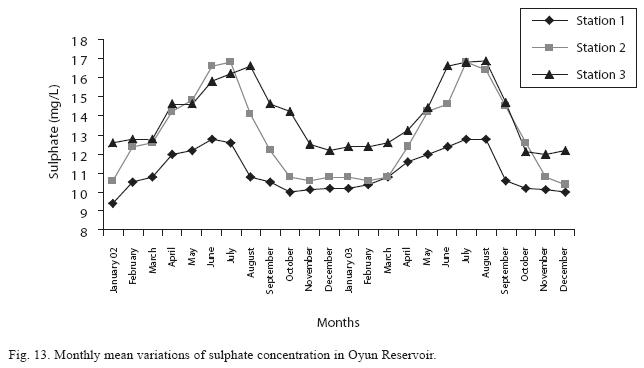
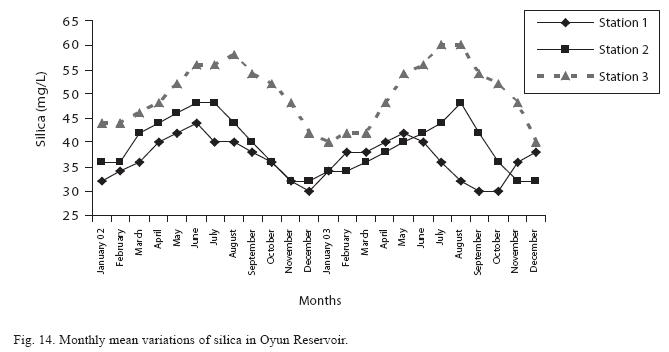
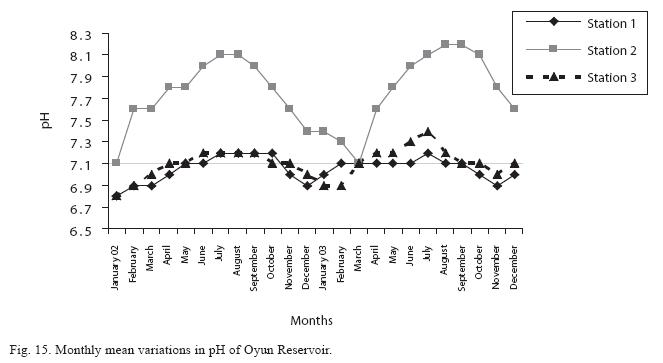
The fluctuations in sulphate concentration are shown in Fig. 13. Sulphate concentration was lowest at 9±0.2mg/L at the beginning of the study in Station 1, it gradually increased until a maximum concentration of 16.9±0.45mg/L was recorded in Station 3. Sulphate was significantly higher in the wet season while the order of higher concentration among the stations was station 3>2>1. No difference occurred within the years. The maximum monthly mean concentration of silica was 60±0.6mg/L recorded from station 3 in July 2003 while station 1 recorded the lowest value of 30±0.2mg/L in December 2002 (Fig. 14). The silica levels were significantly different (P<0.05) among the stations and seasons. Silica was more abundant in the rainy season, and station 3 recorded the highest concentration among the stations. No difference was recorded among the years. The surface water pH fluctuated between slight acidity and moderate alkalinity. The lowest monthly mean pH was 6.8±0.05 obtained at station 3 during the dry season in January 2002, while the highest was 8.2±0.2 obtained from Station 2 in August and September of 2003 (Fig. 15). In the 3 stations, the pH was in the neutral range for most of the study period. No acidic pH was recorded from Station 2; it was either neutral or alkaline for most part of the study. ANOVA (P<0.05) showed pH to be statistically higher during the wet season than in the dry season and pH of Station 2 was significantly higher than the other stations. No difference was noted between the two years.
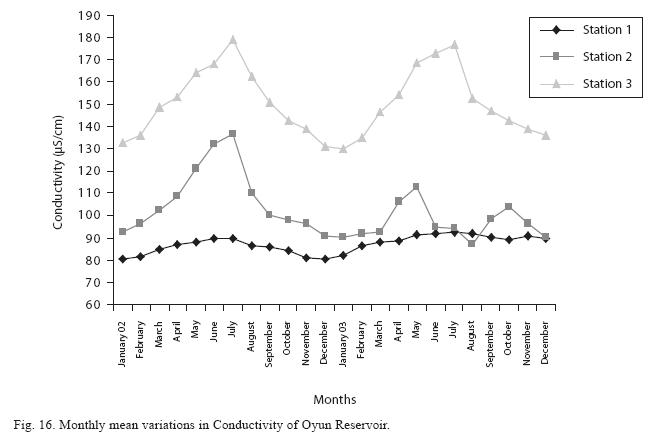
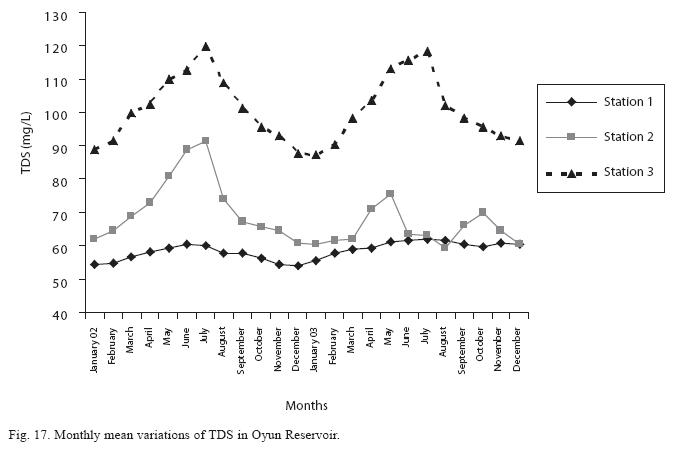
The monthly mean variations in electrical conductivity and total dissolved solids (TDS) followed similar trend. There was slight variation in conductivity and TDS in station 1. The station recorded the lowest value of conductivity (80.4±0.8μS/cm) and TDS (53.9±0.8mg/L) in December 2002 and station 3 recorded the highest variation and concentration of conductivity and TDS with the highest value of conductivity (178.8±2.0μS/cm) and TDS (119.8±2.0mg/L) obtained in July 2002, respectively (Figs. 16 and 17). Both electrical conductivity and TDS showed significant differences in their concentrations among the seasons and stations. The two factors were statistically higher during the rainy season while the order of significant difference between the stations was station 3>2>1.
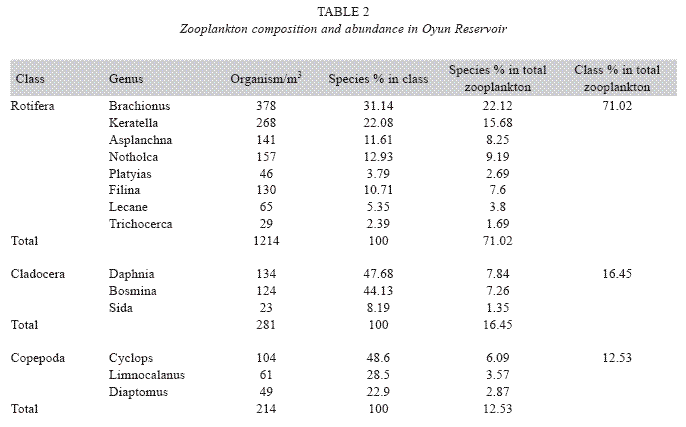
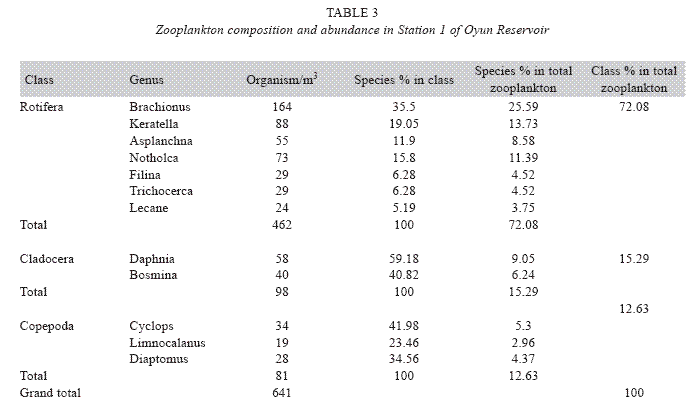
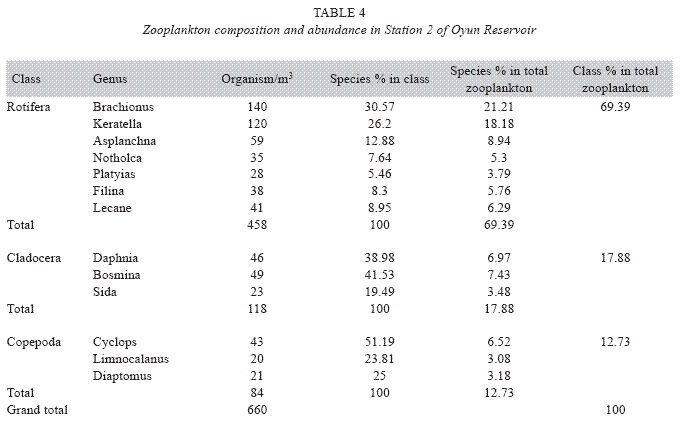
Zooplankton species composition: Fourteen genera of zooplankton were identified from the reservoir. They belong to Rotifera (eight genera), Cladocera three genera) and Copepoda (three genera). The Rotifera (29 to 378 organisms/m3) constituted the largest group making 71.02% of the zooplankton population, this was followed by the Cladocera (16.45%) with organisms ranging between 23 to 124 organims/m3 and Copepoda (12.53%) having density between 49 to 104 organisms/m3. The genus Branchionus dominated the zooplankton genera consisting 22.12% and was also the dominant genus among the Rotifera making 31.14% in the group. The genus Daphnia recorded the highest number among the Cladocera making up 7.84% of the total zooplankton, while the genus Cyclops constituting 6.09% the total zooplankton was the dominant genus among the class Copepoda. The genus Sida, a Cladoceran, was the least abundant consisting only 1.35% among the zooplankton population of Oyun Reservoir. A total of 1709 organisms/ m3 of zooplankton number was recorded in the reservoir (Table 2).
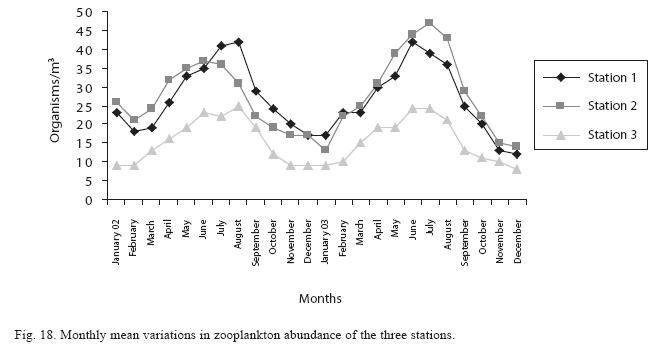
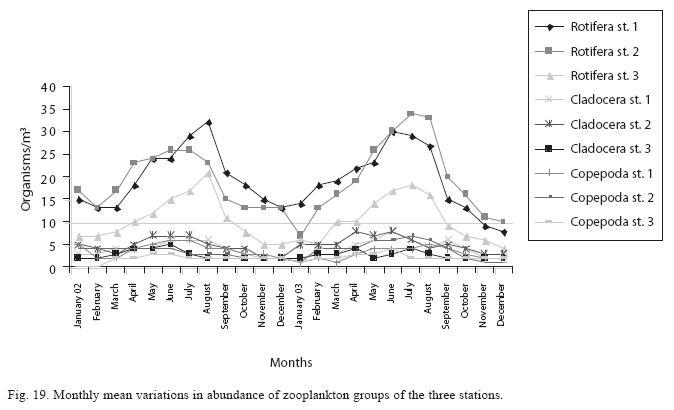
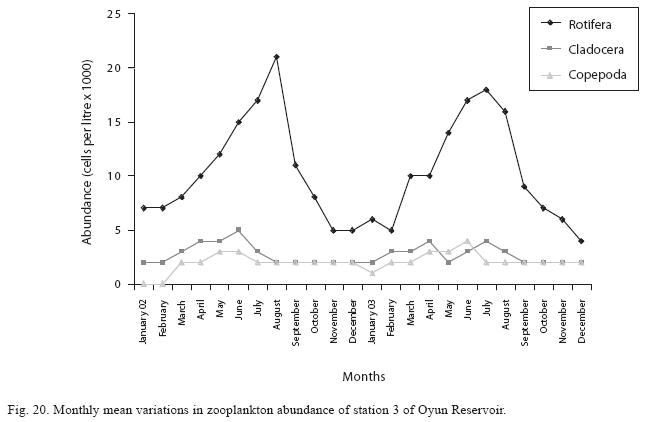
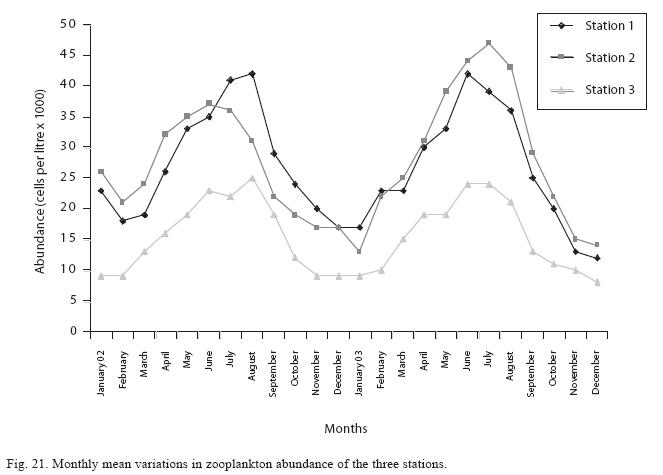
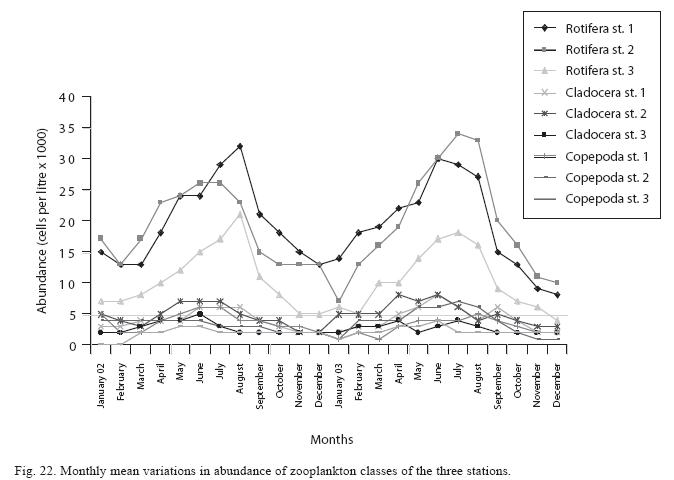
Seasonal abundance and distribution of zooplankton: Seasonal abundance and distribution of the zooplankton in each station is presented in Figures 18, 19 and 20, while their abundance in the three stations is shown in Fig 21. The zooplankton was more prevalent during the rains between the months of April to August, while their population declined during the dry season between November and April. The three stations showed similar genera composition (Fig. 22). The only difference was in their abundance and absence of some genera from one station. A total of 13 genera were recorded from station 1, 12 genera in station 2 and while 10 genera were found in station 3 (Tables 3, 4 and 5).
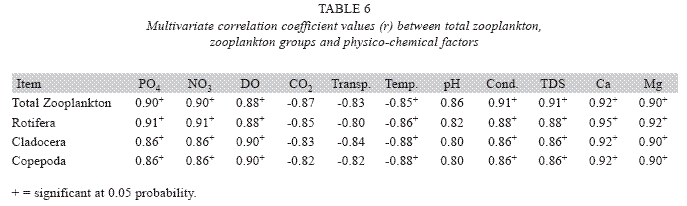
Analysis of variances at P<0.05 showed that total zooplankton density was significantly higher during the wet season than the dry season and station 2 had a higher total zooplankton count than the other stations. No significant difference was seen in the abundance of the zooplankton between the two years. ANOVA (P<0.05) also revealed Rotifera to be significantly abundant in the rains and the order of abundance among the stations was station 1>2>3, while Cladocera and Copepoda were also significantly abundant in the rains and the order of abundance among the stations was station 2>1>3. Multivariate analysis of co-variance (ANCOVA) using PCA and FCA shows total zooplankton and zooplankton groups to be positively correlated (P<0.05) with phosphate, nitrate, dissolved oxygen, conductivity and TDS These factors accounted for the highest correlation for zooplankton abundance in the reservoir. A negative correlation (P<0.05) was observed between total zooplankton and the zooplankton groups with carbon dioxide, transparency, temperature and total alkalinity (Table 6). The correlation of these factors with zooplankton abundance was weak compared to those with significant positive correlations.
Discussion
The zooplankton assemblage in Oyun Reservoir was attributed to several biotic and abiotic factors interacting together. These include temperature, transparency, nutrients, food availability and reservoir morphometry. The fourteen genera of the zooplankton found consisting of Rotifera (eight), Cladocera (three) and Copepoda (three) could not be described as highly diversed. The zooplankton genera found in the reservoir agrees with the observations of Rocha et al. (1999) about zooplankton assemblages in reservoirs. The dominance of Rotifera and genus Brachionus was not unexpected as both the latter and former has been reported by Jeje & Fernando (1986), Egborge & Tawari (1987), Akin-Oriola (2003) and Mustapha & Omotosho (2006) as the most dominant zooplankton group in Nigerian aquatic ecosystems. The high population density of the rotifers could be attributed to their parthenogenetic reproductive patterns and short developmental rate under favourable conditions (Pouriot et al. 1997), their morphological variations called cyclomorphosis and adaptations (Wetzel 2001) and their ability to feed on different food type. The dominance of rotifers was due to its preference for warm waters as highlighted by Dumont (1983) and Segers (2003). The dominance of Brachionus is an indication that the reservoir is eutrophic and their abundance was due to the presence of high organic matter in the reservoir (Matsumura–Tundisi 1999). The relatively low abundance of Cladocera and copepods was as a result of the hydrodynamics of the reservoir such as the low water volume, short residence time, relative old age of the reservoir and its morphometry. The highest population of the two groups occurred at Station 2 which was the transitional zone of the reservoir between the riverine and lacustrine ecosystems. The reason for this was due to the presence of food (phytoplankton) on which they graze and the high transparency of the zone. High clay content and silt turbidity resulting in low transparency was responsible for the low population of the crustacean zooplankton in station 3. The effect of this caused juvenile mortality of the zooplankton and suppressed their growth through food availability. This suppression could have encouraged rotifer population to dominate. Kirk & Gilbert (1990) have noted this type of scenario in a tropical reservoir. The low genera abundance of cladocerans and copepods have also been documented in other water bodies like Lake Cubhu, South Africa (Martin & Cyrus 1994), Ogun and Ona rivers (Akin-Oriola 2003) and Niger-Sokoto River (Jeje & Fernando 1992). The predominance of Daphnia among the cladocerans could have arisen due to its large bodied size which enables it to graze on large quantities and diverse forms of phytoplankton. High Daphnia population occurred due to their effective grazing on rotifers. The density and biomass of cladocerans were primarily determined by food supply. Though, genus Cyclops was the dominant genus among the copepods, its relatively low abundance in the reservoir could be described as a good omen. This is because of the medical implications of large population of the genus in water bodies of Africa, where water is consumed directly from reservoirs without being treated. The low population of the genus could be due to their slow reproduction, growth and renewal rate. Absence of parthenogenetic forms of copepods might be responsible for their low population density.
The high population density and biomass of zooplankton during the rains was traced to high population of phytoplankton food source which were highly abundant within the reservoir during the rains. According to Rocha et al. (1999), increase in primary production (phytoplankton), tends to be followed by increase in zooplankton number and biomass. Muylaert et al. (2003) also corroborated the finding that zooplankton biomass usually reaches their peak during the rains in reservoirs. Apart from food source, low predation by fish during the rains as a result of their breeding could also have encouraged high population of the zooplankton. High fish predation, less availability of food source, low temperature during "harmattan" period could be responsible for the decline in zooplankton during the dry season. Achembach & Lampert (1997) have emphasized these factors as been responsible for zooplankton biomass reduction. Food resource (bottom-up forces) (Carpenter et al. 1987), ability to adapt to food conditions and less predation (topdown forces) (Rosemond et al. 1993) may be the reasons for the significant abundance of Rotifers, Cladocera and Copepoda in the rains. The absence of some genera such as Lecane, Sida and Diaptomus in some stations Could have occurred as a result of patchiness or dispersal. Dispersal has been noted to play a major role in structuring zooplankton population and communities (Shurin & Havel 2002).
The correlations of the zooplankton with nitrate and phosphate may not necessarily be a direct relationship of the zooplankton utilizing the nutrients, but could be attributed to the dependence of the phytoplankton (which serves as food for the zooplankton) on these nutrients. The negative correlation of zooplankton with transparency was as a result of low transparency of the water which hinders zooplankton growth and abundance. High temperature in the dry season may account for the negative correlation with temperature. This observation shows the preference of zooplankton assemblage to low temperature in the reservoir, thus playing a vital role in the zooplankton assemblage of the reservoir. This scenario has been reported by Hulyal & Kaliwa (2007). Alkaline pH was also found to favour zooplankton growth and abundance in the reservoir as seen from the positive correlation with pH. Byars (1960) had reported that zooplankton prefer alkaline waters. Negative correlation of zooplankton with carbon dioxide was due to seasonal influence in which the density of zooplankton decreases during the dry season when carbon dioxide production was in high concentration as a result of decomposition and respiration. Both conductivity and total dissolved solids promoted high zooplankton growth and abundance. This agrees with the findings of Hujare (2005). The water of Oyun Reservoir was soft using the Hanna (2003) hardness scale. The zooplankton assemblage of the reservoir was linked to the soft nature of the water which they prefer as observed in the strong positive correlation with both calcium and magnesium ions which were the contributors of the soft hardness. This type of correlation has been reported by Hulyal & Kaliwal (2007) in Almati Reservoir in India.
The zooplankton community composition of the reservoir also showed the reservoir to be productive and will support a diverse species and population of fishes. The assemblage was strongly influenced by the physico-chemical factors which showed the water quality to be good according to APHA (1998). Temperature, food abundance, nutrients were some of the factors that could limit zooplankton growth, composition and abundance in the reservoir. Maintenance of good water quality in the reservoir will enhance the zooplankton community structure and population dynamics and this will be a great advantage for fish production in the reservoir since the energetic trophic foundations for fish would have been well established.
Resumen
La influencia de las propiedades fisicoquímicas del Reservorio Oyun, Offa, Nigeria (un embalse tropical somero) sobre la composición y abundancia del zooplancton fue investigada en tres estaciones entre enero de 2002 y diciembre de 2003. La diversidad no resultó muy alta con tres grupos de zooplancton: Rotifera con ocho géneros, y Cladocera y Copepoda con tres géneros cada uno. Rotifera dominó (71.02%), seguido de Cladocera (16.45%) y Copepoda (12.53%). El zooplancton fue más común durante la temporada de lluvias, y hubo variaciones en su composición y abundancia a lo largo del embalse. Factores tales como la temperatura, los nutrientes, la disponibilidad de alimentos, la forma y la hidrodinámica del embalse, así como las estrategias reproductivas de los organismos, influyen fuertemente en la composición genérica y la densidad poblacional del zooplancton. La prevención del deterioro ecológico de esta masa de agua, resultaría en un cuerpo de agua más productivo, rico en zooplancton y con mejor pesca.
Palabras clave: zooplancton, Rotifera, Cladocera, Copepoda, embalse, factores físico-químicos.
Received 14-XII-2008. Corrected 27-V-2009. Accepted 23-VI-2009.
References
Achembach, L. & W. Lampert. 1997. Effects of elevated temperatures on threshold food concentrations and possible competitive abilities of differently sized cladoceran species. Oikos 79: 469-476. [ Links ]
Akin-Oriola, G.A. 2003. Zooplankton association and environmental factors in Ogunpa and Ona rivers, Nigeria. Rev. Biol. Trop 51: 391-398. [ Links ]
APHA (American Public Health Association) 1998. Standard methods for the examination of water and waste water. 20th edition. American Public Health Association Inc., New York, USA. [ Links ]
Byars, J.A. 1960. A freshwater pond in New Zealand. Aust. J. Mar Fresh Res. 11: 220-240.
Carpenter, S.R & J.F. Kitchell. 1993. The Trophic Cascade in Lakes. Cambridge University Press, Cambridge, USA.
Carpenter S.R., J.F. Kitchell., J.R.Hodgson., P.A Cochran., J.J. Elser., M.M. Elser., D.M. Lodge., D. Kretchmer., X. He. & C.N. Vonende. 1987. Regulation o f lake primary productivity by food web structure. Ecology 68: 1863-1876. [ Links ]
Dumont, H.J. 1983. Biogeography of rotifers. Hydrobiologia 104: 19-30. [ Links ]
Edmonson, W.T. 1959. Freshwater biology. John Wiley and Sons, New York, USA [ Links ]
Egborge, A.B.M. & P. Tawari. 1987. The rotifers of Warri Rivers, Nigeria. J. Plankton Res. 9: 1-13. [ Links ]
Hach, 2003. Water analysis handbook. Vol. 2: Hach Chemical Company, Loveland, Colorado, USA. [ Links ]
Hanna 2003. The Hanna Catalog. Hanna Instruments Inc. Woonsocket RI, USA. [ Links ]
Hujare, M.S. 2005. Hydrobiological studies on some water reservoirs of Hatkanangale Tahsil (Maharashtra). Ph.D Thesis, Shivaji University, Kolhapur, India. [ Links ]
Hulyal,S.B & B.B. Kaliwal. 2007. Water quality assessment of Almatti reservoir of Bijarpur (Karnataka State, India) with special reference to zooplankton. Environ. Monit. Assess. 139: 29-306. [ Links ]
Jackson, D.C & E.H. Schmitz. 1987. Zooplankton abundance in vegetated and non-vegetated areas: implications for fisheries management. Proceedings of Southeastern Association of Fish Wildlife Agencies 41: 214-220. [ Links ]
Jeje, C.Y. & C.H. Fernando. 1986. A practical guide to the identification of Nigerian zooplankton (Cladocera, Copepoda and Rotifera). Kanji Lake Research Institute, New Bussa, Nigeria Publication. Nigeria. [ Links ]
Jeje, C.Y. & C.H. Fernando. 1992. Zooplankton association in the middle Niger- Sokoto Basin (Nigeria- West Africa). Int. Revue ges. Hydrobiol. 77: 237-253. [ Links ]
Kirk, K.L. & J.J. Gilbert. 1990. Suspended clay and the population dynamics of planktonic rotifers and cladocerans. Ecology 71: 1741-1755. [ Links ]
Margalef, R. 1976. Limnology of the Spanish reservoirs. Publication No. 123, Centro de Estudios Hidrográficos, Madrid, Spain. [ Links ]
Martin, T.J & D.P. Cyrus. 1994. Zooplankton in the open water of Lake Cubhu, a freshwater coastal lake in Zululand, South Africa. Wat. S. Afr. 20: 107-112. [ Links ] Matsumura-Tundisi, T. 1999. Diversidade de zooplâncton em represas do Brasil, pp. 39-54. In R Henry (ed.). Ecologia de reservatórios: estrutura, função easpectos sociais. FUNDIBIO/FAPESP, Botucatu, Brazil. [ Links ]
Mustapha, M.K. & J.S. Omotosho. 2006. Hydrobiological studies of Moro Lake, Ilorin, Nigeria. Nig. J. Pure & Appl. Sci. Vol 21: 1948-1954. [ Links ]
Mustapha, M.K. 2008: Assessment of the water quality of Oyun Reservoir, Offa, Nigeria, using selected physico-chemical parameters. Turk. J. Fish. Aquat. Sci. 8: 309-319. [ Links ]
Muylaert, K., S. Declerck., V. Geenens., J.V. Wichelen., H. Deegans., J. Vandekerkhove., K.V. Gucht., N. Vloemans., W. Rommens., D. Rejas., R., Urrutia., K. Sabbe., M. Gills., K. Decleer., L.D. Meester. & W. Vyverman. 2003. Zooplankton, phytoplankton and the microbial food web in two turbid and two clear shallow lakes in Belgium. Aquat. Ecol. 37: 137-150. [ Links ]
Pourriot. R., C. Rougier, & A. Miquelis. 1997. Origin and development of river zooplankton: example of the Marne. Hydrobiologia 345: 143-148. [ Links ]
Rocha, O., T. Matsumura-Tundisi., E.L.G. Espindola., K.F. Roche & A.C. Rietzler. 1999. Ecological theory applied to reservoir zooplankton, p. 29-51. In Tundisi, J.G & M. Straskraba (eds.). Theoretical reservoir ecology and its applications. International Institute of Ecology, Brazilian Academy of Sciences. Backhuys Publishers, Leiden, Holland. [ Links ]
Rosemond, A.D., P. Mulholland & J.W. Elwood. 1993. Top-down and Bottom-up control of stream periphyton: effects of nutrients and herbivores. Ecology 74:1264-1280. [ Links ]
Sampaio, E.V., O. Rocha., T. Matsumura-Tundisi & J.G. Tundisi. 2002. Composition and abundance of zooplankton in the limnetic zone of seven reservoirs of the Paranapanema River, Brazil. Brazil J. Biol. 62: 525-545. [ Links ]
SAS Institute. 2003: SAS/Stat User’s Guide, Version 9.1.3. SAS Institute Inc., Carry, North Carolina, USA. [ Links ]
Segers, H. 2003: A biogeographical analysis of rotifers of the genus Trichocerca Lamarck, 1801 with notes on taxonomy. Hydrobiologia 500: 103-114. [ Links ]
Shurin, J.B. & J.E. Havel. 2002. Hydrologic connections and overland dispersal in an exotic freshwater crustacean. Biol. Invasions. 4: 431-439. [ Links ]
Wetzel, R.G. 2001. Limnology: lake and river ecosystems Academic Press, New York, USA. [ Links ]
Whitford, L.A. & G.J. Schumacher. 1973. A manual of freshwater algae. Sparks Press, Raleigh, North Carolina, USA. [ Links ]












 uBio
uBio 
 .
.