Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.57 n.3 San José Sep. 2009
Composition and abundance of zooplankton groups from a coral reef lagoon in Puerto Morelos, Quintana Roo, Mexico, during an annual cycle
José N. Álvarez-Cadena1, Uriel Ordóñez-López2, Alma Rosa Almaral-Mendivil1 & Amira Uicab-Sabido2
1. Universidad Nacional Autónoma de México, Instituto de ciencias del mar y limnología, Unidad académica Puerto Morelos, Apdo. Postal 1152, Cancún, Quintana Roo, C. P. 77501, México, inac@mar.icmyl.unam.mx
2. Instituto politécnico nacional, Centro de investigación y estudios avanzados, Unidad Mérida. Km 6 antigua carretera a Progreso. A. P. 73 CORDEMEX, C.P. 97310, uriel@mda.cinvestav.mx
Abstract: Zooplankton sampling was carried out monthly from January to December 1990 at station A near the coastline, and station B near the reef barrier, in a tropical coral reef lagoon in the Mexican Caribbean Sea. Samplings were made at midnight, near surface, with a conical net (mouth 0.40 m, mesh 330 µm) for 10 min. Salinity varied from 35.1 to 36.3 psu and temperature from 26.3 to 30.2 ºC. The Bray-Curtis test applied to these results has defined two seasons: the dry season from November to May, and the wet season from June to October. A total of 37 zooplankton groups were found. Copepods were the most abundant contributing 49.0% of the total capture with Acartia espinata, Calanopia americana and Farranula gracilis as the most numerous. In the total zooplankton, however, cirripeds captured in only 15 samples of 24 were second in abundance (20.9%). Decapods, present all year-round and more abundant during the wet season, were third and contributed 19.2%. The rest of the groups were scarce and only amphipods (2.4%) and larvaceans (2.0%) were relatively abundant. The abundance of captured organisms correlated with the abiotic factors measured, thus, in the dry season, abundance was lower (mean 7.3 orgs/m3), while in the wet season the mean catch was 36.8 orgs/m3. Rev. Biol. Trop. 57 (3): 647-658. Epub 2009 September 30.
Key words: copepods, zooplankton, coral-reef lagoon, Puerto Morelos, Mexican Caribbean Sea.
Caribbean waters are generally regarded as oligotrophic, but reefs and associated lagoon systems in these areas are also well known for their remarkable high productivity (Nybakken 1993, Sorokin 1995). This high productivity has its origin, in part, from the large beds of submerged vegetation such as the sea grass Thalassia testudinum Banks ex König, Syringodium sp.; Halodule sp.; macroalgae, phytoplankton and surrounding mangroves. Vegetation is not, however, the only contributor to the high content of organic matter in reef lagoon systems. A great variety of products from animal origin such as discarded exoskeletons from crustaceans, "houses" of appendicularians and excretions in the form of fecal pellets, metabolic by-products from fish or gelatinous material from the reefs them- selves also contribute importantly (Vidal 1980, Fenaux 1985). Other events are also respon sible for the high organic matter content in reef lagoon areas; thus, tides, currents, winds, promote increments not only the input of nutrients, but also in the horizontal and vertical transport of planktonic organisms (Thompson and Golding 1981, Wolansky and Pickard 1983).
Studies on the zooplankton communities in the Mexican Caribbean Sea are scarce and discontinuous, implying a lack of sufficient information of this important community. In the reef barrier lagoon of Puerto Morelos, Suárez-Morales and Gasca (1990) studied the dial variation of zooplankton associated with T. testudinum. Similarly, Álvarez-Cadena et al. (1998), produced information on the copepod fauna, Ramírez-Ávila and Álvarez-Cadena (1999) on the monthly variation of chaetognaths, and Ramírez-Ávila (2001) and Álvarez-Cadena et al. (2007) on ichthyoplankton.
The Puerto Morelos reef lagoon waters due to an extremely narrow continental shelf, allows the presence of open marine waters in the area, resulting in very homogeneous salinity and temperature. The lagoon is presently a National Park where fishing activities and tourist facilities are restricted and supervised by Government and tourism authorities. On the other hand, this area known as The Riviera Maya is having substantial increments in human population settlements. Our monitoring on the zooplankton community (since 1990) will provide valuable information regarding the previous environmental conditions, as changes are taking place. Thus, the present study will be important as an antecedent for planktonic fauna of the reef lagoon.
This study is the first work in its kind where monthly changes in the composition and abundance of the zooplankton groups during an annual cycle (1990) are reported. Results are related with temperature and salinity variations along the year in the coral reef lagoon of Puerto Morelos, in the Quintana Roo State of Mexico.
Materials and methods
The Puerto Morelos coral reef lagoon is located ca. 35 km south of Cancun City, on the Yucatan Peninsula at 20º51´ N and 85º55´ W. The area has a narrow continental shelf with dominant northward marine currents that eventually pass through the Yucatan Channel. Dominant east-southeast trade winds blow most of the year except in winter with northern winds ("Nortes") (Merino and Otero 1990). The climate, according to García (1988), is sub-humid, with heavier rains in summer and an annual mean temperature higher than 22 ºC.
Sampling was carried out monthly in conditions of darkness (midnight) from January to December 1990 at two stations within the reef lagoon. One of the stations was located near the coastline (A) and the other close to the coral reef barrier (B). Captures were made for 10 min. following a circular path with a conical plankton net of 0.40 m diameter and 1.20 m length (mesh 330 µm), carrying a flowmeter for estimating animals density (orgs/m3). Salinity was measured with a Beckman salinometer and temperature with an immersion thermometer. Samples were fixed with buffered formaldehyde and seawater was added to obtain a 4% solution (Smith and Richardson 1979). The biological material was analyzed with the help of a stereomicroscope. Samples were not analyzed whole, instead, sub samples were made using a Folsom splitter, and in every one of them at least 800 animals were counted and identified making sure that the aliquot was representative of the whole sample (Omori and Ikeda 1994). Groups identification was made following Tregouboff and Rose (1957) and Bolstovskoy (1999), while Campos hernandez and Suarez-Morales (1994) were consulted for the copepod species reported. One-way ANOVA and Bray-Curtis statistical analyses were carried out with ANACOM (de la Cruz 1994).
Results
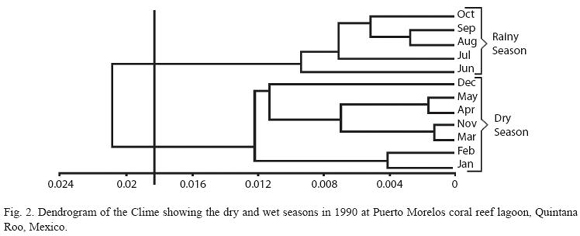
Temperature and salinity were not significantly different between the two stations (p>0.05), thus, they were grouped together and mean values are provided (Fig. 1). Temperature varied from 30.2 ºC (July) to 26.3 ºC in January (annual mean for both sites was 28.1 ºC). Salinity was homogeneous, highest values were recorded in July (36.3 psu) and lowest in December (35.1 psu) (annual mean for both stations was 36 psu). The Bray-Curtis test applied to these results showed two seasons, the dry period from November to May and the rainy season from June to October (Fig. 2).
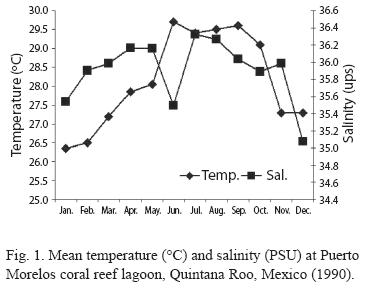
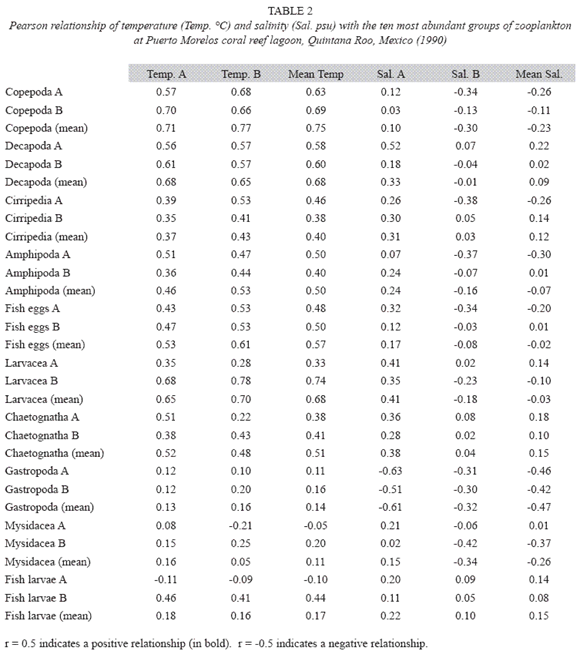
According to the total catch of organisms, the zooplankton community can be separated into two seasons. One from November to April, 29.5 36.4 characterized by low captures with a mean density of 7.3 orgs/m3, and the other from May to October (mean density 36.8 orgs/m3) (Fig. 3). A total of 37 zooplankton groups were recorded (Table 1), with copepods collected at all times and contributing 49.0% of the total. The ten most abundant groups can be seen in Fig. 4 and the Pearson relationship of them with temperature and salinity at stations A and B (and mean) in Table 2. Several organisms hada relationship with temperature (in bold), but not with salinity.
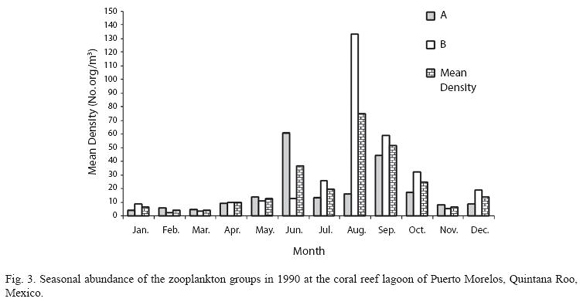
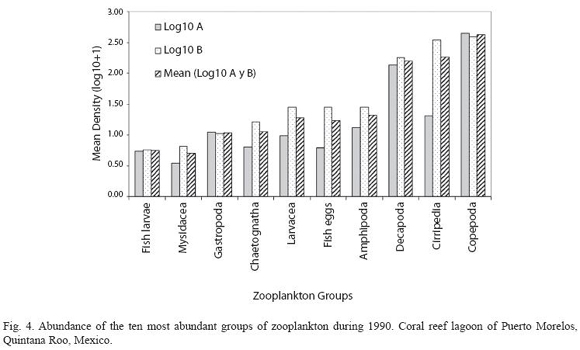
Acartia espinata Esterly was the most abundant copepod at both sampling sites. highest catches of this group occurred at station A in June (2 033.8 orgs/m3) and September (1 469.7 orgs/m3) and also in September at station B (1 425.1orgs/m3). Cirripeds, although collected only on 15 samples out of 24, were second in abundance (20.9%), with higher numbers at station B in August and September (3 718.1 and 444.9 orgs/m3, respectively). Decapods, representing 19.2% of the total, were third in abundance and collected in all samples, with highest catches at station A in August (342.8 orgs/m3) and September (213.3 orgs/m3) and at station B, in August, September and October (390.1 orgs/m3, 292.6 orgs/m3 and 566.3 orgs/m3) respectively. Amphipods (2.4%) and appendicularians (2.0%) were fourth and fifth in abundance, both being more abundant at station B in August with 140.2 orgs/m3 and 97.5 orgs/m3 respectively. The rest of the groups made up less than 10% (Table 1).
Both sampling places registered a similar number of taxa, at station A 35 groups were obtained (Cephalopoda and Nudibranchia were absent), and 34 at station B (Sergestidae, Turbellaria and hemichordata were lacking), these groups were the less abundant and frequently captured.
Discussion
Stability of the marine conditions at the coral reef lagoon of Puerto Morelos is possibly due to the combined effect of 1) a narrow continental shelf, substantially allowing the intrusion of oceanic water, and 2) the fact that continental runoff is limited, in spite of fresh water discharges through underground water wells. Thus, salinity and temperature varied little throughout the year as previously reported by other authors (Merino and Otero 1990, Álvarez-Cadena et al. 1998).
As a generality, the abundance of the groups captured correlated with the climatic seasons. Thus, higher abundance was recorded from June through October, peaking in August-September (rainy season), and fewer animals were caught from November to May (dry season). The high abundance of zooplankton during the wet season is possibly due to the runoff of micronutrients from the land, resuspension of sediments originated by strong winds promoting phytoplankton production and eventually a high abundance in zooplankton.
Results presented in this study have dissimilarities with the work of Alvarez-Cadena et al. (2007), as these authors covered a larger area and included the Sistema Lagunar Nichupte (SLN) with particular environmental conditions (this body water has been reported as nearly "choked", with a long residence time of its waters and with some characteris tics of eutrophication). Thus, A. tonsa Dana (copepoda) and Ferosagitta hispida (Conant) (Chaetognatha) were most abundant there, it was found 41 zooplankton groups and reported, because of the records in the SLN, lower salini- ties and temperatures.
Copepods were the most abundant group, as is usually the case in most zooplankton samples whether in Mexico or for other parts of the world. Álvarez-Cadena et al. (1998), reported that highest densities of these holoplanktonic animals occurred in September. In other closed or semi-enclosed lagoon systems, whether on the Pacific or Atlantic coasts of Mexico, extreme dominance of A. tonsa is common (Lee and McAlice 1979, Durbin et al. 1983, Álvarez-Cadena and Cortés-Altamirano 1990). This high dominance of A. tonsa was also observed at the Nichupté Lagoon system (located in the surroundings of the hotel zone of Cancun) by Álvarez-Cadena et al. (1996), and Álvarez-Cadena and Segura Puertas (1997) (in both studies dominance was higher than 80%). In the present study, however, for the reef lagoon of Puerto Morelos, A. spinata was found as dominant, particularly in September (rains). Other important species were: Calanopia americana Dahl, Farranula gracilis (Dana) and Clausocalanus furcatus (Brady). Álvarez-Cadena et al. (1998), also reported these species as recurrently abundant for the copepod fauna in the lagoon, and also contributing importantly for other two stations in the adjacent sea (beyond the coral reef barrier).
Although not collected in all samples, cirripeds were the second most abundant group. Barnacles, were initially found in May, reached its maximum in August-September and declined in the following months. Álvarez-Cadena et al. (1996) in a preliminary study at the Nichupté Lagoon System, some 35 km north of Puerto Morelos, recorded barnacles only during the rainy season (with higher temperatures) and none during the dry season. Castellanos and Suárez-Morales (1997), working in the Mahaual area, some 300 km to the south, did not record cirripeds, sampling being carried out in December-January. Thus, it seems plausible that these animals reproduce when temperatures start to increase, peaking during the summer.
Decapods, collected at all times, were third in abundance with increasing numbers in July and reaching a maximum in November. According to Monrroy-Velazquez (pers. comm.) adult crabs of Cardisoma guanhumi Latreille and Gecarcinus lateralis Freminville, are inhabitants of the swamps and mangrove areas of Puerto Morelos, reproduce around the rainy season and are commonly seen migrating to the sea for spawning. Castellanos and Suárez-Morales (1997) from the Mahaual reef, and Gasca and Castellanos (1993) for the Chetumal Bay, both reported decapod larvae as dominant. Monrroy-Velazquez (2000) reported that Caridea, Anomura and Brachiura are the most important decapods in the submerged vegetation in the reef lagoon, with major captures during summer (rainy season when higher temperatures prevail) and lowest in winter.
Amphipods and larvaceans (collected at all times), were fourth and fifth in abundance respectively. In the case of the amphipods, Suarez-Morales and Gasca (1990), and Morales and Murillo (1996), reported frequent captures of benthic amphipods. The high frequency in the capture of this taxon in our work is probably related with the shallowness of the Puerto Morelos lagoon, and it is plausible to expect the frequent integration of the epibenthos into the water column, particularly at those times when disturbance of sediment is possible, as usually occurs during rains or strong winds. In our samples it was possible to observe that the most abundant and frequent amphipods were Gammaridae, reported as dwelling in shallow coastal areas, and related in the process of making detritus. Typically oceanic amphipods were extremely rare or absent in most of the samples.
Appendicularians are one of the most abundant and frequent zooplankters in coastal tropical environments (Alldredge 1972, 1976, Sato et al. 2001). They are reported as an important food-link and as common prey for chaetognaths and fish larvae (Feigenbaum and Maris 1984). Furthermore, it is also known that their "houses" are used as a food source or as a surface habitat by other microorganisms (Ohtsuka and Kubo 1991, Steinberg 1995, Mochiocha and Iwamizu 1996). These urochordates have short generation times, and the importance of these animals as contributors to the high productivity in reef areas has been recognized. Sato et al. (2001) reported a generation time for "houses" of Oikopleura dioica between 6 (at 15 ºC) and 3 days (at 25 ºC).
In this study, Larvaceans abundance was probably underestimated as they possibly went through the net mesh because of their small size.
Although collected in lower numbers and less frequently, other taxa followed the same general pattern of abundance, i.e. higher abundance in summer and lower in winter. Reports of the dominant chaetognath species in the area Ferosagitta hispida and Flaccisagitta enflata (Grassi) (Ramirez-Avila and Alvarez-Cadena 1999) and fish larvae (Ordóñez-Lopez, pers. comm.) showed they were also more abundant during the rainy period.
It is important to mention that this study carried out in 1990, is the first work made monthly during an annual cycle for the area. More recent studies by Alvarez-Cadena, et al. (2007) (samples collected during 2004), reported changes for the zooplankton in the area, and it seems that at the present, highest abundance has been found for winter (January-February), instead than for summer as reported in this contribution.
Resumen
Se efectuaron muestreos mensuales de zooplancton en el Caribe de México de enero a diciembre de 1990 en dos estaciones en la laguna arrecifal de Puerto Morelos en el estado de Quintana Roo, una situada cerca de la línea de costa (A), y la otra próxima a la barrera de coral (B). Los arrastres se realizaron en condiciones de oscuridad (a medianoche), cerca de la superficie durante 10 min con una red cónica de boca de 0.40 m y malla de 330 micras. La salinidad varió de 35.1 a 36.3 ups y la temperatura de 26.3 a 30.2 oC. La prueba de Bray-Curtis definió dos épocas en el año, la de secas de noviembre a mayo, y la de lluvias de junio a octubre. Se capturaron un total de 37 grupos de zooplancton. Los copépodos, capturados en todas las muestras, contribuyeron con 49% del total en donde las especies Acartia espinata, Calanopia americana y Farranula gracilis fueron las más abundantes. Los cirripedios aún capturados únicamente en 15 ocasiones de 24, contribuyeron con 20.9% y fueron mayormente abundantes en la estación cerca de la barrera de arrecife. Los decápodos, presentes en todos los muestreos, contribuyeron con 19.2%. Otros grupos menos importantes fueron: anfípodos (2.4%) y larváceos (2.0%). La abundancia general del zooplancton coincide en general con las épocas del año, teniendo mayor abundancia durante la temporada de lluvias (36.8 org./ m3 en promedio) y menores capturas durante las secas con 7.3 org/m3 en promedio.
Palabras clave: copépodos, zooplancton, laguna arrecifal, Puerto Morelos, Caribe de México.
References
Alldredge, A. 1972. Abandoned larvacean house: A unique food source in the pelagic environment. Science 177: 885-887. [ Links ]
Alldredge, A. 1976. Discarded appendicularian houses as sources of food, surface habits, and particulate matter in planktonic environments. Limnol. Oceanogr. 21: 14-23. [ Links ]
Álvarez-Cadena, J.N. & R. Cortés-Altamirano. 1990. Algunos factores físicos y Biológicos que afectan las poblaciones naturales de Acartia tonsa y A. Lilljeborgii (COPEPODA: ACARTIIDAE) en el estero de Urías, Sinaloa, México. Invest. Mar. CICIMAR 5: 147-153. [ Links ]
Álvarez-Cadena, J.N., M.E. Islas-Landeros & E. Suárez-Morales. 1996. A preliminary zooplankton survey in a Mexican eutrophicated coastal lagoon. Bull. Mar. Sci. 58: 694-708. [ Links ]
Álvarez-Cadena, J.N., U. Ordóñez-López, A.R. Almaral-Mendivil, M. Ornelas-Roa & A. Uicab-Sabido. 2007. Larvas de peces del litoral arrecifal del norte de Quintana Roo, Mar Caribe de México. Hidrobiológica 16: 107-120. [ Links ]
Álvarez-Cadena, J.N. & L. Segura-Puertas. 1997. Zooplankton variability and copepod species assemblages from a tropical coastal lagoon. Gulf. Res. Rep. 9: 345-355. [ Links ]
Álvarez-Cadena, J.N., E. Suárez-Morales & R. Gasca. 1998. Copepod assemblages from a reef-related environment in the Mexican Caribbean Sea. Crustaceana 71: 411-433. [ Links ]
Bolstovskoy, D. 1999. South Atlantic Zooplankton. Backhuy, Leiden. [ Links ]
Campos-Hernández, A. & E. Suárez-Morales. 1994. Copépodos Pelágicos del Golfo de México y Mar Caribe. I. Biología y Sistemática. Centro de Investigaciones de Quintana Roo, México. [ Links ]
Castellanos, I. & E. Suárez-Morales. 1997. Observaciones sobre el zooplancton de la zona arrecifal de Mahaual, Quintana Roo (Mar Caribe Mexicano). An. Inst. Biol. Univ. Nal. Autón. México. Serie Zoología 68: 237-252 [ Links ]
De la Cruz, A.G. 1994. ANACOM: Sistema para el análisis de comunidades, versión. 3.0 Manual del usuario. CINVESTAV-IPN Mérida, México. [ Links ]
Durbin, E.G., A.G. Durbin, T.J. Smayda & P.G. Verity. 1983. Food limitation of production of adult Acartia tonsa in Narragansett Bay, Rhode Island. Limnol. Oceanogr. 28: 1199-1213 [ Links ]
Feigenbaum, D. & R.C. Maris. 1984. Feeding in the Chaetognatha. Oceanogr. Mar. Biol. Ann. Rev. 22: 343-392 [ Links ]
Fenaux, R. 1985. Rhythm of secretion of oikopleurid´s houses. Bull. Mar. Sci. 37: 498-503. [ Links ]
García, E. 1988. Modificaciones al sistema de Köppen para adaptarlo a las condiciones de la Republica Mexicana. Instituto de Geografía. Universidad Nacional Autónoma de México, México D.F., México. [ Links ]
Gasca, R & I. Castellanos. 1993. Zooplancton de la Bahía de Chetumal, Mar Caribe de México. Rev. Biol. Trop. 41: 619-625. [ Links ]
Lee, W. & B.J. McAlice. 1979. Seasonal succession and breeding cycles of three species of Acartia (Copepoda: Calanoida) in a marine estuary. Estuaries 2: 228-236. [ Links ]
Merino, M. & L. Otero. 1990. Atlas ambiental costero de Puerto Morelos, Quintana Roo. CONACyT-UNAM. Centro de Investigaciones de Quintana Roo, México [ Links ]
Mochiocha, N. & M. Iwamizu. 1996. Diet of anguilloid larvae: leptocephali feed selectively on larvacean houses and fecal pellets. Mar. Biol.125: 447-452. [ Links ]
Monrroy-Velazquez, V. 2000. Variaciones en la composición y abundancia en la fauna de decápodos asociados a pastizales marinos en el Caribe Mexicano. Tesis de Maestría. Inst. Cienc. Mar Limnol., México. [ Links ]
Morales, A.R. & M.M. Murillo. 1996. Distribution, abundance and composition of coral reef zooplankton, Cahuita National Park, Limón. Costa Rica. Rev. Biol. Trop. 44: 619-630. [ Links ]
Nibakken, W.J. 1993. Marine Biology: An Ecological Approach. 3rd Ed. harper Collins College, New York, USA. [ Links ]
Ohtsuka, S. & N. Kubo. 1991. Larvaceans and their houses as important food for some pelagic copepods. Bull. Plankton Soc. Japan. Spec. Vol.: 535-551. [ Links ]
Ramírez-Avila, Y. 2001. Ictioplancton arrecifal frente a Puerto Morelos, Quintana Roo, durante la época de lluvias: Patrón a fina escala de la estructura de la comunidad, ensamblajes y su relación con el medio. Tesis de Maestría. Centro de Investigación y Estudios Avanzados del Instituto Politécnico Nacional, Unidad Mérida, Mérida, México. [ Links ]
Ramírez-Ávila, Y. &. J.N. Álvarez-Cadena. 1999. Chaetognath species composition from a coral reef lagoon in the Mexican Caribbean Sea. Rev. Biol. Trop. 47: 157-163. [ Links ]
Sato, R., Y. Tanaka, & T, Ishimaru. 2001. House production by Oikopleura dioica (Tunicata, Appendicularia) under laboratory conditions. J. Plankton Res. 23: 415-423. [ Links ]
Smith, P.E & S.L. Richardson. 1979. Técnicas modelo para prospecciones de huevos y larvas de peces pelágicos. FAO. Documento Técnico de Pesca 175. [ Links ]
Sorokin, Y.I. 1995. Coral Reef Ecology. Springer, Germany. [ Links ]
Steinberg, D.K. 1995. Diet of copepods (Scopalatum vorax) associated with mesopelagic detritus (giant larvacean houses) in Monterey Bay, California. Mar. Biol. 122: 1606-1620. [ Links ]
Suárez-Morales, E. & R. Gasca. 1990. Variación dial del zooplancton asociado a praderas de Thalassia testudinum en una laguna arrecifal del Caribe Mexicano. Univ. Cienc. 7: 57-64. [ Links ]
Thompson, R.O. & T.J. Golding. 1981. Tidally induced ¨upwelling¨ by a Great Barrier Reef. J. Geoph. Res. 86C: 6517-6521. [ Links ]
Tregouboff, G. & M. Rose. 1957. Manuel de Planctonologie Mediterraneenne. Centre National de la Recherche Scientifique, Paris, France. [ Links ]
Vidal, J. 1980. Physioecology of zooplankton. II. Effects of phytoplankton concentration, temperature, and body size on the development and molting rates of Calanus pacificus and Pseudocalanus sp. Mar. Biol. 56: 135-146. [ Links ]
Wolansky, E. & L. Pickard. 1983. Upwelling by internal tides and Kelvin waves at the continental shelf break on the Great Barrier Reef. Aust. J. Mar. Freshwater Res. 34: 65-80. [ Links ]














