Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.57 n.1-2 San José Mar./Jun. 2009
Paternal behavior and testosterone plasma levels in the Volcano Mouse Neotomodon alstoni (Rodentia: Muridae)
Juana Luis1, Lorena Ramírez1, Agustín Carmona2, Guadalupe Ortiz3, Jesús Delgado4 & René Cárdenas2
1. Unidad de Morfología y Función, FES Iztacala, Universidad Nacional Autónoma de México, Av. de los Barrios No. 1, Tlalnepantla, Edo. de México, México; luisdc@correo.unam.mx
2. Departamento de Biología Celular, Facultad de Ciencias, Universidad Nacional Autónoma de México, 04510 México, D. F.; agustincarmona@correo.unam.mx; rcv@fciencias.unam.mx
3. Laboratorio de Biología de la Reproducción Humana, Hospital Juárez de la Secretaría Salubridad. Avenida Instituto Politécnico Nacional, Magdalena de la Salinas México D. F., México; gortizl@prodigy.net
4. Psicología Animal Experimental, FES Iztacala, Universidad Nacional Autónoma de México, Av. de los Barrios No. 1, Tlalnepantla, Edo. de México, México; jdsr@correo.unam.mx
Abstract: Paternal behavior and testosterone plasma levels in the Volcano Mouse Neotomodon alstoni (Rodentia: Muridae). Although initially it was thought that testosterone inhibited the display of paternal behavior in males of rodents, it has been shown that in some species high testosterone levels are needed for exhibition of paternal care. In captivity, males of volcano Mouse (Neotomodon alstoni) provide pups the same care provided by the mother, with the exception of suckling. Here we measured plasmatic testosterone concentrations 10 days after mating, five and 20 days postpartum, and 10 days after males were isolated from their families in order to determine possible changes in this hormone, associated to the presence and age of pups. Males of volcano Mouse exhibited paternal behavior when their testosterone levels were relatively high. Although levels of this hormone did not change with the presence or pups age, males that invested more time in huddling showed higher testosterone levels. It is possible that in the volcano Mouse testosterone modulates paternal behavior indirectly, as in the California mouse. Rev. Biol. Trop. 57 (1-2): 433-439. Epub 2009 June 30.
Key words: paternal behavior, testosterone, volcano Mouse, Neotomodon alstoni, wild rodents.
In most mammals only the female takes care of her young; however, in 10% of the genera of these vertebrates the male provides some care to its pups. Paternal care has been observed in primates, carnivorous and rodents (Kleiman and Malcolm 1981, Cantoni 1993, Tardifi 1997).
In rodents paternal care has been associated to the mating system and social organization. The degree of caring provided by the male to its pups is related to the probability of paternity, and therefore, paternal care is more frequent in monogamous species than in promiscuous ones (Kleiman and Malcolm 1981). In such cases, male rodents supply the same care to the offspring as females, except for suckling (Dudley 1974, Gubernick and Alberts 1987). Indeed, the presence of the male during lactation increases the survival and growth of the offspring (Clutton-Brock and Godfray 1991, Cantoni and Brown 1997, Luis et al. 2000).
Although it has been established that the initiation of maternal behavior is elicited by hormonal changes that occur at the end of pregnancy and that its maintenance depends on the presence of the young (Numan 1988, Rosenblatt et al.1998), little is known about the hormonal bases of the paternal behavior. The first studies on this subject pointed out to a hormonal control of this behavior; for example, it was reported that prolactin promotes the exhibition of paternal cares in males of biparental mammals (Brown et al. 1995, Ziegler and Snowdon 1997, Reburn and Wynne-Edwards 1999). Steroid hormones, such as testosterone, estradiol and progesterone have also been related to paternal behavior, although their effects have not been widely studied and results from these studies have been inconsistent (Wang and De Vries 1993, Clark and Galef 1999, Lonstein and De Vries 1999, Hume and Wynne-Edwards 2005). Nonetheless, it has been shown that males exhibiting paternal cares present hormonal changes that facilitate the spreading of this behavior. It is possible then that in the regulation of this behavior, the same neuroendocrine circuits regulating maternal behavior are present (Wynne-Edwards and Reburn 2000).
Correlative studies in the Mongolian gerbil (Meriones ungiculatus) and the dwarf hamster (Phodopus campbelli), have found an increase in plasma levels of testosterone in the male, before female delivery, followed by a decrease after the young are born (Brown et al.1995, Clark et al.1997, Reburn and Wynne-Edwards 1999). In humans it has also been observed a decrease in testosterone levels when they become fathers (Berg and Wynne-Edwards 2001; Burnham et al. 2003, Fleming et al. 2002). Testosterone decrease has been interpreted as evidence of an inhibitory effect on the paternal behavior by this hormone. However, in other mammals like the male cotton-top tamarin (Saguinus oedipus) and the Californian mouse (Peromyscus californicus) high testosterone levels are needed to display paternal cares (Ziegler and Snowdon 2000, Trainor and Marler 2001). In the latter, it was also found that progesterone plasma levels were lower in fathers than in sexually inexperienced males (Trainor et al. 2003).
Similarly to the previously mentioned species, males of the volcano Mouse (Neotomodon alstoni), an endemic rodent of the Mexican Transvolcanic Belt, exhibit paternal care in captivity, and its presence during the suckling period increases growth and survival of the young (Luis et al. 2000, 2004). Here we assess plasmatic levels of testosterone at different stages between mating, parturition and isolation of males from the female and offspring, in order to relate them to the exhibition of paternal behavior in the presence and age of the young. This study is the first report on the hormonal basis of the paternal behavior of this species.
Materials and methos
The volcano mice used in the present study were obtained from a colony kept at the Bioterium of the Faculty of Sciences, UNAM. Animals were originally captured at the Sierra del Ajusco, between kilometers 44 and 45 of the Mexico City to Cuernavaca free highway. The 14 males used in the study had weights between 45-55 g and were maintained at a light-dark cycle 12:12 hr and ambient temperature between 17-21ºC, in acrylic cages (32 x 23 x 15 cm) with sawdust bedding. They were fed Purina Nutricubes for small rodents and tap water ad libitum; and the diet was supplemented with carrots every other day.
These individuals had previous sexual experience and were monogamically mated. A single observer recorded the following parental activities from birth up to 21 days postpartum: huddling, sniffing, grooming, retrieving of the young and maintenance of the nest. Observations of parental behavior were daily performed at approximately 50 cm from the cage, during 30 min between 11:00 am and 3:00 pm.
Blood samples for testosterone analysis were taken from males at 10 days after mating, and during the suckling period at day 5 (when the male is most active in caring of the young) and at day 20 (when paternal care have diminished). After the last blood sampling the males were isolated from the family, placing them in individual cages in a contiguous room, and a last blood sample was taken after 10 days of isolation. Blood samples (100µl) were obtained from the retrorbital senus with heparinized capillaries, under light ether anesthesia. Plasma was separated by centrifugation and stored at -70ºC until testosterone analysis by Radioimmunoassay (RIA). RIA was performed by duplicate with a Coat-A-Count kit for Total Testosterone (Cat# PITKTT, Diagnostic Products Corporation, Los Angeles, CA, USA), with 125I testosterone and sensitivity of 0.04 ng/m. Inter- and intra-assay coefficients variation were 5.4 and 13.45 %, respectively. The radioactivity was measured with a gamma counter model 1282 Compugamma (LKB- Wallac, Turku, Finland).
The Animal Care Unit of "Facultad de Ciencias, Universidad Nacional Autónoma de México", provided assistance during the laboratory work, which followed the American Society of Mammalogists guidelines for animal care and use (Animal Care and Use Committee 1998), as well as the Mexican federal regulations for institutional animal care units ("Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación" 2001).
The obtained data for huddling, sniffing and grooming were grouped in four day blocks and plotted. Spearman correlations between these activities and age of young were performed.
Since there was considerable variation in plasmatic testosterone magnitudes, logarithmic transformations were made before analysis by one way (ANOVA). Spearman correlations were also made between testosterone concentrations at days 5 and 20 postpartum and the time spent in huddling by males, respectively. Statistic analyses were performed using software GPower (version 3.0.10 for Windows).
Results
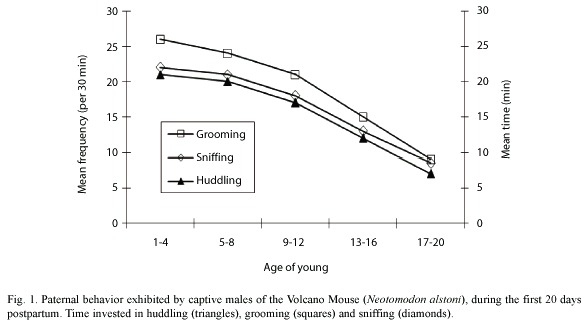
All males actively participated in the care of young during the 20 postpartum days. The main paternal activities were huddling, grooming, sniffing, retrieval of the young and maintenance of the nest. Among these, the first three were the ones observed with higher frequency (Fig 1). The time that the males invested in huddling significantly correlated with the age of young (r = -.53, n = 92, P<0.05, Fig. 1). Likewise, the frequency with which the males groomed ( r =-73, n = 91, P<0.05, Fig. 1) and sniffed (r = -.57, n = 128, P<0.05, Fig.1) the young, also correlated with age.
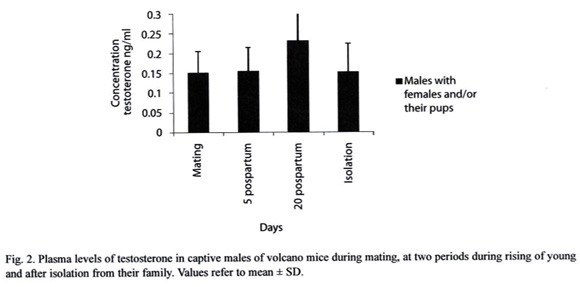
Plasmatic testosterone levels of the males were 0.15 ± 0.05 ng ml-1 at day 10 after mating, 0.15 ± 0.06 ng ml-1 and 0.23 ± 0.20 ng ml-1 at days 5 and 20 of the suckling period, respectively. Ten days later these males were isolated from their families and testosterone levels were 0.15 ± 0.07 ng ml-1 (Fig. 2). No significant differences were found among testosterone levels with relation to any of the sampled times (F = 0.80, g. l. = 3/52, P = 0.49). Testosterone concentration of males at day 5 postpartum only correlated positively with the time spent in huddling, but at day 20 postpartum no correlation was found (day 5, r = .73, P<0.5; day 20, r = -0.35, P>0.5).
Discussion
All males exhibited paternal behavior during the first 20 days postpartum that they were kept with the pups. This conduct was composed of the following activities: huddling, grooming, sniffing and retrieving the pups. Age of the pups had a strong influence over the time spent by males in huddling, grooming and sniffing the pups. Males displayed more paternal activity during the first postpartum days, confirming our previous report (Luis et al. 2000).
Males of Volcano mouse that provided paternal care to their pups, during the nursing period, presented testosterone plasma levels as high as when they were mating. These results differ from those found in the Mongolian gerbil and Dwarf hamster, whose testosterone levels decreased after their offspring was born (Brown 1995, Reburn and Wynne-Edwards 1999). Although in the latter species, another study reported that testosterone levels did not decrease when the pups were born, which does not support the hypothesis that in male rodents the levels of this hormone would be low when males take care of their pups. Nonetheless, male Dwarf hamsters that always retrieved the pups had lower testosterone levels than those that never retrieved them (Schum and Wynne-Edwards 2005). Likewise, males of the marmoset (Callithrix kuhlii) exhibited high rates of pup retrieving when their urinary testosterone levels were low (Nunes et al. 2001). On the other hand, in other mammalian males, like the cotton-top tamarin, the urinary testosterone level increases throughout the pregnancy and remain elevated during the nursing period, when males give parental care to their pups (Ziegler and Snowdon 2000). In the California mouse it was demonstrated that testosterone maintains paternal behavior. In this rodent the castration reduced the degree of paternal care, while the castrated males that received testosteronereplacement therapy displayed higher levels of paternal behavior (Trainor and Marler 2001).
Here, testosterone levels in males of captive Volcano mouse, did not show any significant change associated with the presence of the pups. Similarly, in male marmosets, testosterone levels changed after the pups were born, independently of the presence of the young (Nunes et al. 2000). Therefore, it is probably that more than the pups presence itself, the presence of the female is the stimulus that maintains paternal behavior in the Volcano mouse. In fact, cohabitation of the male with female in the Mongolian gerbils, causes neuroendocrine changes in the former that inhibit its infanticide behavior promoting, instead, paternal behavior; moreover, it was observed that plasmatic testosterone levels were not modified in males when they were separated from the pups during four hours (Brown et al.1995). In males of the California mouse, it was observed that chemosensory stimulus from the female promotes paternal behavior when the pups are not present (Gubernick and Alberts 1987).
Plasmatic testosterone levels did not decrease in the volcano mouse when exhibiting paternal behavior. Indeed, a positive correlation between levels of this hormone and the amount of paternal care provided by the male was observed at day 5 postpartum. It is possible that testosterone is required for displaying paternal behavior in the volcano mouse, such as in the California mouse (Trainor and Marler 2001). In this rodent testosterone does not have a direct effect in paternal behavior, but it does have an indirect one through its conversion to estrogen (Trainor and Marler 2002, Trainor et al. 2003). The same indirect effect of testosterone might occur in the volcano mouse, but only future experimental studies such as castration models with testosterone-replacement, will contribute to establish the actual role of testosterone in the paternal behavior of this rodent.
Acknowlegments
The Animal Care United of "Facultad de Ciencias, Universidad Nacional Autónoma de México", provided assistance during the laboratory work of this project. F. Chiang made valuable suggestions to early drafts. This work was supported by PAPCA 2007, FES Iztacala, UNAM.
Resumen
Aunque inicialmente se pensaba que la testosterona inhibía el despliegue de conducta paterna en los machos de roedores, en algunas especies se ha demostrado que son necesarios niveles altos de testosterona para la exhibición de cuidados paternos. En cautiverio, los machos del ratón mexicano de los volcanes (Neotomodon alstoni), proporcionan a sus crías los mismos cuidados que la madre, con la excepción del amamantamiento. En este estudio se midieron los niveles plasmáticos de testosterona: en el día 10 del apareamiento, 5 y 20 días postparto, y 10 días después de aislar a los machos de sus familias, para establecer si los niveles de esta hormona cambian con relación a la presencia y edad de las crías. El ratón de los volcanes exhibió cuidados paternos cuando sus niveles de testosterona fueron relativamente altos, aunque los niveles de esta hormona no variaron significativamente con relación a la edad y presencia de las crías. Sin embargo, los machos que invirtieron más tiempo en abrigar a las crías, presentaron concentraciones relativamente más altas de testosterona. Es posible que en el ratón de los volcanes la testosterona regule la conducta paterna de manera indirecta, como ocurre con el ratón de California.
Palabras clave: conducta paterna, testosterona, ratón de los volcanes, Neotomodon alstoni, roedores silvestres.
References
Animal Care and Use Committee. 1998. Guidelines for the capture, handling, and care of mammals as approved by the American Society of Mammalogists. Journal of Mammalogy. 79:1416–1431. [ Links ]
Berg, J. S. & E.K. Wynne-Edwards. 2001. Changes in testosterone, cortisol and estradiol levels in men becoming fathers. Clin. Proc.76: 582-592. [ Links ]
Brown, R. E., T. Murdoch, P. R. Murphy & W. H. Moger. 1995. Hormonal responses of male gerbils to stimuli from their mate and pups. Horm. Behav. 29: 474-491. [ Links ]
Burnham, T. C., J. F.Chapman, P. B. Gray, M. H. McIntyre, S. F. Lipson & P. T. Ellison. 2003. Men in committed, romantic relationships have lower testosterone. Horm. Behav. 44: 119-122. [ Links ]
Cantoni, D. & R. E. Brown. 1997. Paternal investment and reproductive success in the California mouse, Peromyscus californicus. Anim. Behav. 54: 377-382. [ Links ]
Cantoni, D. 1993. Hormonal and experiential factors influencing parental behavior in male rodents: an integrative approach. Behav. Proc. 30: 1-28. [ Links ]
Clark, M. M., D. V. Desousa & B. G. Galef. 1997. Parenting and potency: alternative routes to reproductive success in male Mongolian gerbils. Anim. Behav. 54: 635-642. [ Links ]
Clark, M. M. & B. G. Galef. 1999. A testosterone-mediated trade-off between parental and sexual effort in male Mongolian gerbils (Meriones unguiculatus). J. Comp. Psychol. 113: 388-395. [ Links ]
Clutton-Brock, T. & C. Godfray. 991. Parental investment, pp. 235-262. In J. R. Krebs & N. B. Davies (eds.). 3rd Behavioral Ecology, ed., Blackwell Scientific Publications. Oxford. [ Links ]
Dudley, D. 1974. Contributions of parental care to the growth and development of the young in Peromyscus californicus. Behav. Biol. 11: 155-166. [ Links ]
Fleming, A. S., C. Corter, J. Stallings & M. Steiner. 2002. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Horm. Behav. 32:85-98. [ Links ]
Gubernick, D. J. & J. R. Alberts. 1987. The biparental care system of the California mouse, Peromyscus californicus. J. Comp. Psych. 101: 169-177. [ Links ]
Hume, M. J. & E. K. Wynne-Edwards. 2005. Castration reduces male testosterone, estradiol and territorial aggression, but not paternal behavior in biparental dwarf hamsters (Phodopus campbelli). Horm. Behav. 48: 303-310. [ Links ]
Kleiman, D. G. & J. R. Malcolm. 1981. The evolution of male parental investment in mammals, pp. 347-387. In D. J. Gubernick & P. H. Klopfer (eds.). Parental care in mammals. Plenum Press, New York. [ Links ]
Lonstein, J. S. & G. J. De Vries. 1999. Sex differences in the parental behaviour of adult virgin prairie voles: independence from gonadal hormones and vasopressin. J. Neuroendocrinol. 11: 441-449. [ Links ]
Luis, J., A. Carmona, J. Delgado, F. A. Cervantes & R. Cárdenas. 2000. Parental behavior of the volcano mouse Neotomodon alstoni (Rodentia:Muridae), in captivity. J. Mammal. 81(2): 600-605. [ Links ]
Luis, J., F. A. Cervantes, M. Martínez-Torres, R. Cárdenas, J. Delgado & A. Carmona 2004. Male influence on maternal behavior and offspring of captive volcano mice (Neotomodon alstoni) from México. J. Mammal. 85(2): 268-272. [ Links ]
Numan, M. 1988. Maternal Behavior, pp.1569-1654. In E. Knobil, J. Neill, L. Ewing, G. Greenwald, C. Markert & D. Pfaff (eds.).The Physiology of reproduction. Raven Press, New York. [ Links ]
Nunes, S., J. E. Fite & J. A. French . 2000. Variation in steroid hormones associated with infant care behaviour and experience in male marmosets (Callithrix kuhlii). Anim. Behav. 60: 857-865. [ Links ]
Nunes, S., J. E. Fite, K. J. Patera & J. A. French. 2001. Interactions among paternal behavior, steroid hormones, and parental experience in male marmosets (Callithrix kuhlii). Horm. Behav. 39: 70-82. [ Links ]
Reburn, C. J. & K. E. Wynne-Edwards. 1999. Hormonal changes in males of naturally biparental and uniparental mammals. Horm. Behav. 35: 163-176. [ Links ]
Rosenblatt, J. S., A. Olufowobi & H. I. Siegel. 1998. Effects of pregnancy hormones on maternal responsiveness, responsiveness to estrogen stimulation on maternal behavior, and the lordosis response to estrogen stimulation. Horm. Behav. 33: 104-114. [ Links ]
Secretaría de Agricultura, Ganadería, Desarrololo Rural, Pesca y Alimentación. 2001. Norma Oficial Mexicana NOM-062-ZOO-1999, Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. Diario Oficial de la Federación. 75:113–160. [ Links ]
Schum, E. & E. K. Wynne-Edwards. 2005. Estradiol and progesterone in paternal and non-paternal hamsters (Phodopus) becoming fathers: conflict with hypothesized roles. Horm. Behav. 47: 410-418. [ Links ]
Tardifi, D. S. 1997. The bioenergetics of parental behavior and the evolution of alloparental care in marmosets and tamarins, pp. 11-28. In N. G. Solomon & J. A. French (eds.). Cooperative Breeding in Mammals Cambridge University Press, Cambridge. [ Links ]
Trainor, C. B. & A. C. Marler. 2001. Testosterone, paternal behavior, and aggression in the Monogamous California Mouse (Peromyscus californicus). Horm. Behav. 40: 32-40. [ Links ]
Trainor, C. B. & A. C.Marler. 2002. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc. R. Soc. London . B 269: 823-829. [ Links ]
Trainor, C. B., I. M. Bird, N. A. Alday, B. A. Schlinger & C. A. Marler. 2003. Variation in aromatase activity in medial preoptic area and plasma progesterone is associated with the onset paternal behavior. Neuroendocrinol. 78:36-44. [ Links ]
Wang, Z. & G. J. De Vries. 1993. Testosterone effects on paternal behavior and vasopressin immunoreactive projections in prairie voles (Microtus ochrogaster). Brain Res. 631: 156-160. [ Links ]
Wynne-Edwards, E. K. & C. J. Reburn. 2000. Behavioral endocrinology of mammalian fatherhood. TREE 15: 464-468. [ Links ]
Ziegler, T. E. & C. T. Snowdon. 1997. Role of prolactin in paternal care in a monogamous New World primate, Saguinus oedipus. Ann. New York Acad. Sciences. [ Links ]
Ziegler, T. E. & C. T. Snowdon. 2000. Preparental hormone levels and parenting experience in male cottontop tamarins, Saguinus oedipus. Horm. Behav. 30: 59-167. [ Links ]














