Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.56 n.4 San José Dec. 2008
Reproduction of the fish Poeciliopsis gracilis (Cyprinodontiformes: Poeciliidae) in Coatetelco, a tropical shallow lake in Mexico
José Luis Gómez-Márquez1, Bertha Peña-Mendoza1, Isaías H. Salgado-Ugarte2, Abby K. Sánchez-Herrera1 & Leonardo Sastré-Baez1
1. Laboratorio de Limnología, F.E.S. Zaragoza, U.N.A.M. Batalla 5 de Mayo esq. Fuerte de Loreto, Ejército de Oriente, C.P. 09230 Iztapalapa, México, D.F.; lgomez@servidor.unam.mx
2. Laboratorio de Biometría y Biología Pesquera, F.E.S. Zaragoza, U.N.A.M. Batalla 5 de Mayo esq. Fuerte de Loreto, Ejército de Oriente, C.P. 09230 Iztapalapa, México, D.F.; isalgado@servidor.unam.mx
Abstract: A reproductive analysis of 1 225 specimens of Poeciliopsis gracilis obtained through monthly samples from Coatetelco, a tropical shallow lake in Central Mexico, was made. There was an evident sexual dimorphism, including a difference in body size at the onset of reproduction. Sex ratio deviated significantly from unity. Monthly variations in gonadosomatic (GSI), hepatosomatic (HSI) indexes and ovarian development stages showed that the spawning season was from July to October, coinciding with the rainy season and phytoplankton biomass increase. The largest sizes were 50 mm for females and 43 mm for males. Rev. Biol. Trop. 56 (4): 1801-1812. Epub 2008 December 12.
Key words: chloropyll "a", gonadosomatic index, hepatosomatic index, Poeciliopsis gracilis, reproduction, sex ratio, Mexico.
The members of the fish order Cyprinodontiformes are cosmopolitan in tropical and temperate latitudes. The genus Poeciliopsis belongs to the Heterandrini Tribe and comprise 44 species. The poeciliids are small fishes, none attains an overall length of 50 mm and most are less than half of that size (Rosen and Bailey 1963, Rauchenberger 1989, Huidobro 2000). Only a small proportion of the nearly 200 poeciliids species has been studied in an ecological context (Meffe and Snelson 1989). The porthole livebearer Poeciliopsis gracilis (Heckel 1848) is of Central American origin (Meffe 1989, Miller et al. 2005).
The species is a member of the live-bearing teleost poeciliids and can be found in the Pacific slope from the Río verde in Mexico, to the Eastern tributaries of the Fonseca Gulf in Honduras (Choluteca River) and, possibly Nicaragua. On the Atlantic slope it is found from the Coatzacoalcos River in Mexico to Nicaragua Lake (Miller 1966, Miller et al. 2005). P. gracilis has been recently recorded to the North of its previously known distribution zone at the Balsas River basin in Morelos and Puebla, Mexico (Mejia 1992).
Poeciliids live in a broad array of habitats, occupying temperate to tropical zones, desert, rivers, lakes springs, fresh and brackish marshes, seacoasts, and saline mangrove swamps. However, basic ecology of most poeciliids is unknown. Considering the economic importance of poeciliids (aquarium trade, biological control, conservation) few species have been studied in the field as P. gracilis (Huidobro 2000) and P. infans (Galindo-villegas and Sosa-Lima 2002); the majority of the available information comes from laboratory studies (Meffe and Snelson 1989).
Martínez et al. (2004) and Maya et al. (2006) mentioned that ornamental fish culture is one of the main economic activities at the Morelos State, being the Poeciliidae family the most cultivated at the fish farms, with an annual production of 16.5 millions of fry that represent US$2.59 millions.
P. gracilis is a species which is caught at several aquatic systems in Mexico. Its ecologically very tolerant, inhabiting in quiet water of streams, flood-water ponds, lagoons, micro reservoirs, lakes and dams, in water clear to turbid or very muddy (Meffe and Snelson Jr. 1989, Miller et al. 2005). This species starts to be reared in laboratory in order to obtain results about to how the environmental condition affects its survival and how the food amount and quality, affects its growth rate and fecundity.
In spite of the high demand of P. gracilis as a forage species in Mexico, its commercial value is very low, as from the ecological point of view there are few investigations about the impact that this introduced species causes on the native fauna and habitats in different countries.
To date, no studies in relation with P. gracilis reproduction has been carried out at Coatetelco, a tropical shallow lake at Morelos, Mexico. The purpose of this study was to analyze the relationships between the reproductive cycle (fecundity-length, fertility-length, size of first reproduction, sex ratio, maturity development stages, etc.) of P gracilis and the environmental conditions.
Materials and methods
Study site: Lake Coatetelco (18°45 N; 99°20 W) is located at an altitude of 1100 m in Morelos State (Anonymous 1981, Anonymous 1998). It has a surface area of approximately 120 ha, a mean depth of 0.49 m and a maximum depth of 1.5 m. Gómez-Márquez (2002) cited that the conductivity varies between 574 and 1 110 µsiems/cm, pH values were considered as lightly alkaline (8.9 average), even that at a global way the tendency was to increase from June to December. Lake Coatetelco is characterized by a sub humid warm climate, with annual mean temperature between 24°C and 26°C. The highest air temperatures were recorded in May (32°C) and the lowest in December and January (20 -21°C). The observed water temperature fluctuation in this study was similar to that reported by Granados (1990), in that low temperatures prevailed in January and February, whereas high temperatures prevailed during the rest of the year. Water level decreased between January and September.
Monitoring: The samples were taken approximately at monthly periods from May 2002 to April 2003. Fish were caught with a seine 10 m long and 1.0 m deep, with 5 mm mesh and fixed in 10 percent formaldehyde. Specimens were then transported to the laboratory where they were measured to the nearest 0.1 cm and weighed to the nearest 0.01 g. Maturation stages for females were established by gonadal inspection following Mendozas technique (1962) and Contreras-MacBeath and Ramírez-Espinoza (1996) description. The ovaries were removed, weighed and the ovules, eggs and embryos from each female caught throughout the study were counted.
The gonadosomatic and hepatosomatic indexes (GSI and HSI respectively) were estimated by:
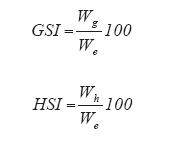
where Wg is gonad wet weight (g), Wh is liver wet weight and, We is eviscerated wet weight (g). This can be considered as a measurement of reproductive cost. To establish the size of first reproduction for females, the ovaries were examined following the criteria employed by Reznick and Miles (1989) and using the logistic equation (King 1995). For males, we considered the smallest sample with completely formed gonopodial structures.
The total length (Lt)-total weight (Wt) relationship was calculates by regression between these variables for each sex using the formula:
Wt = aLt b
A covariance analysis made it possible to determine whether or not there were significant differences at p<0.05, for the length-weight relationship between both sexes.
Condition (K) was calculated for males and females using the Fultons formula (Nikolsky 1963):
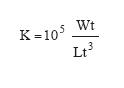
Where Wt = total wet weight (g) and Lt = total length (mm). The Kruskal-Wallis test (p<0.05) was used to test whether condition differed between males and females.
Fecundity was estimated by calculating the regression of the ovules number on the total length, using logarithms. The sex ratio was analyzed for the whole population by month. The statistical significance of the monthly ratio results was established by a goodness of fit Chi-squared test (χ²).
Some environmental factors were also investigated to determinate if they are associated with the breeding cycle of the fish. Surface temperature was measured using a thermometer around 12:00 h during each sampling occasion. In addition, previously measured month water temperature data for the period between 2002 and 2003 were considered. A photosynthetic pigment (chlorophyll"a") was measured by means of the spectrophotometric method (Wetzel and Likens 1991).
The catalogue number of voucher specimens is IBUNAM 12725.
Results
Biometry: The total sample size was 1 225 P. gracilis individuals. Standard body length ranged from 19 to 43 mm (males) and from 20 to 50 mm (females) with weights between 0.08 to 0.72 g (males) and 0.1 to 5.0 g (females). The females were larger than males (t-student = 13.98; p<0.05). There was an evident sexual dimorphism in the morphological features of P. gracilis, being the modification of the anal fin in males to form a gonopodium the main sexual distinctive characteristic.
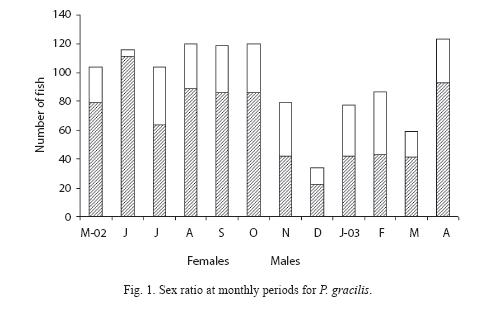
Sex ratio: Of the 1 143 adults specimens dissected, 345 (30.2%) were males and 798 (69.8%) were females. The sex ratio of the catch as a whole was 2.3:1 (female:male), significantly different from a 1:1 ratio (χ²= 89.76, p<0.05). The females dominated significantly throughout the year, except February when more adult specimens were males (Fig. 1).
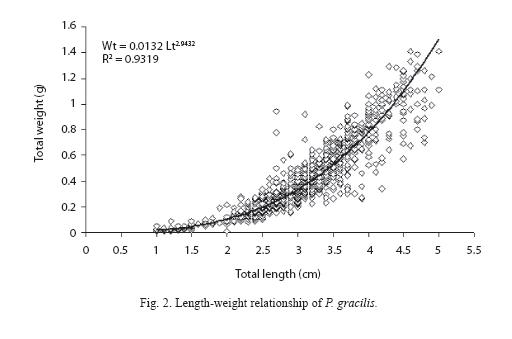
Length-weight relationship: This relation was evaluated for all individuals, and for each sex separately (females and males), due to the significant difference between the male and female slopes of Wt-Lt regressions (ANCOVA; F = 19.03, p<0.05). These equations are given below:
Females:
Wt = 0.0134 Lt 2.94, R² = 0.9431, p<0.05
Males:
Wt = 0.0155 Lt 2.73, R² = 0.7426, p<0.05
All fishes:
Wt = 0.0132 Lt 2.94, R² = 0.9319, p<0.05
Weight increased allometrically with size (Fig. 2), since b value had a significant difference from 3 (t = -2.027, p<0.05 for females, and t = -8.530, p<0.05 for males). For the same length, females were heavier.
Maturation stages and reproduction: The size of first reproduction for females was 28 mm total length. In males, the smallest specimen identified with completely formed gonopodial structure measured 22 mm total length.
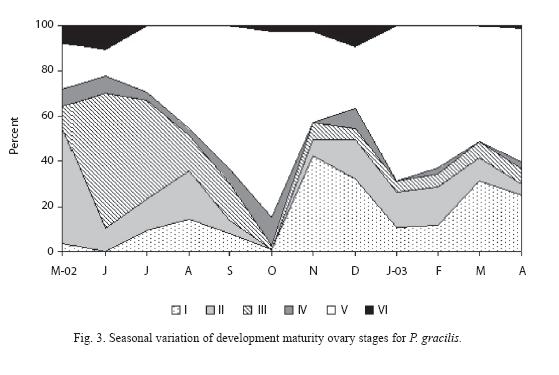
Percentage of each gonadic development stage is illustrated in Fig. 3. Monthly occurrences in the ovarian maturation stages of P. gracilis were used to establish the breeding period of the species. According with females gonadic maturation stages, 14.6 percent of the total fishes were first reproduction (II), 15.4 percent were maturing (III), 4.6 percent were mature (IV), 46.9 percent were ripe (V) and, 2.8 percent were spent (VI). Therefore, 54.2 percent of the fish were in the reproductive process. In the males all gonads analyzed were mature all the time.
We found a broad breeding activity as interpreted by the presence of development stages IV and V, which were present all the year. Fig. 3 shows that for females, the highest proportion of the average gonadal ripe stage (V) was present in October. However, from January to April another reproduction peak was registered. On the other hand, the fishes in spent stage (VI) were observed in June and in December.
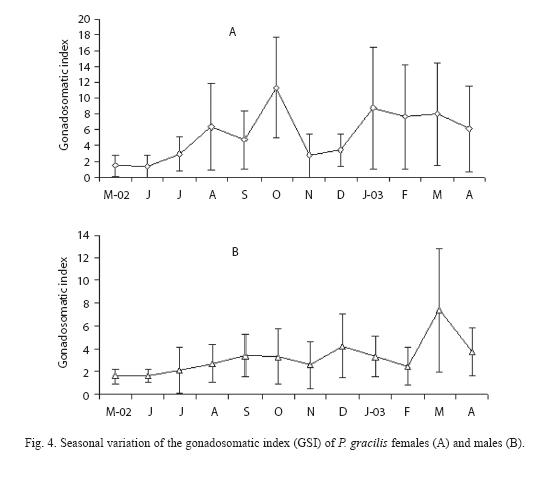
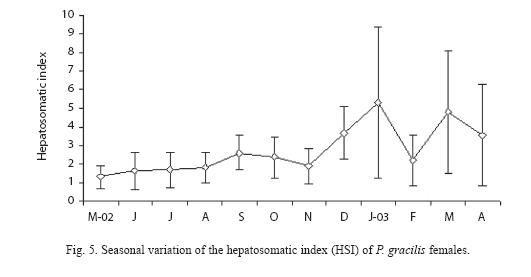
For females (Fig. 4A), the highest gona dosomatic index (GSI) values occurred in October and decreased during November, reaching another peak in March. The GSI of males (Fig. 4B) varied over the year and significant differences between months were found (Kruskal-Wallis, H = 82.29, p<0.05), with March and December showing the highest values. A similar pattern of gonad and liver weight means for females was detected. The value of hepatosomatic index (HSI) of females, reached its maximum in September (Fig. 5).
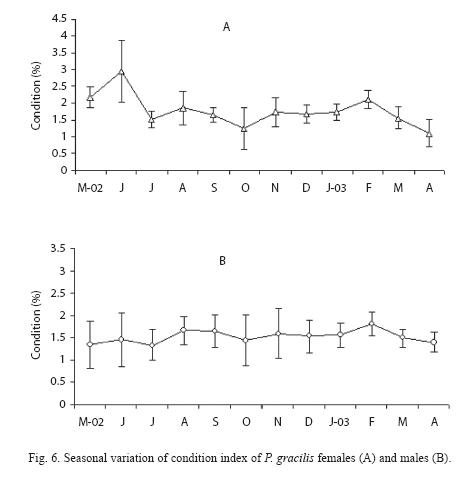
With regard to condition, there were not significant differences between sexes (Kruskal-Wallis, H = 3.20, p>0.05), with females (Fig.6A) always in better condition than males (Fig.6B), and showing similar pattern. The condition values of males calculated with total or eviscerated weight were similar. Condition decreased from February to June, especially in the females, maybe due to prevailing adverse environmental conditions. When the reproductive cycle began in July, the condition of both sexes improved, although it can be considered that in the females the energetic cost of embryo formation is higher. In October the maximum condition values were achieved, as a consequence of weight increase due to embryo growth and to the favorable environmental conditions.
The fecundity (number of oocytes + number of embryos) was estimated from 455 females and ranged from 1 to 74, with a mean value of 44. There were no correlation between fecundity and body length (r=0.46, p>0.05). Moreover, embryos from each female caught throughout the study were counted (fertility). Brood size was variable too, ranging from one to 12, with a mean fertility of four. We calculated a regression between fertility and total length, which resulted in a significant (p<0.05) determination coefficient (r²) of 5.04%, indicating that the number of embryos is not influenced by female size.
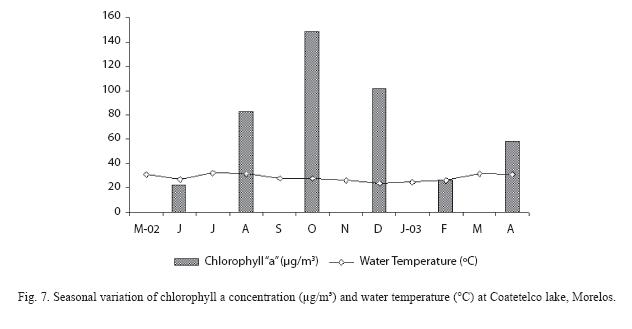
Environmental factors: Data on water temperature and chlorophyll "a" are presented in figure 7. During the present study, the area was characterized by a dry (November to May) and a rainy (June to October) season. The lake water level increased from July to October in response to the "heavy rains". The water temperature ranged from 24.1°C to 32.3°C, and was relatively high from March to October. Low temperatures were recorded from December to January. Respect to chlorophyll "a", the data showed that phytoplankton biomass peaks occurred during October and April.
Discussion
Due to the limited information about reproduction biology of P. gracilis in different journals, the discussion here was elaborated considering papers on biological and reproductive aspects occurring with other species or genera.
During the present study we confirmed that P. gracilis is a dimorphic species with marked secondary sexual differences in size and the presence of a gonopodium in males, a characteristic shared by all poeciliids (Rodriguez 1977). Porthole livebearer females live longer and reach larges sizes than males, possibly because in general, females take a longer time to mature and continue growing throughout their life. Males stop growing, or grow very little after the gonopodium has been completely formed and they do not live as long after reaching maturity (Snelson 1984, 1989). Reznick (1983) point out that high growth rates favors the survival of young fishes because, if they are bigger, then they can feed on a large range of prey and successfully avoid predation, so decreasing their mortality rate.
Another aspect that could also have some influence on survival is the size at first reproduction, because males are precocious compared to females, which have a shorter growth period prior to maturation and therefore lower growth rates. Contreras-MacBeath and Ramírez-Espinoza (1996) mentioned that males take advantage of being precocious and also prolong their reproductive life, while females delay maturation but they are able to bear and nourish a greater number of larger embryos.
The sex ratio significantly female biased (1:2.3) was in accordance with what has been found in other poeciliids species such as Poecilia sphenops (1:5) (Martínez-Trujillo 1983) and Gambusia holbrooki (1:4) and this is a well known feature occurring in sexually dimorphic fishes, particularly those belonging to the Poeciliid family (vargas and de Sostoa 1996). Contrary to this, Urriola et al. (2004a) cited that the sex ratio for Poecilia reticula was significantly male biased.
Snelson (1989) mentioned that the preponderance of females might be attributed to the differential mortality of the sexes. Females have higher survivorship rates due to their larger sizes, longer lifespan, less conspicuous colors, and that they are more resistant to the rigors of reproductive effort and unfavorable environmental conditions. Rosenthal et al. (2001) cited that the brightly colored appears detrimental for survival and involves a higher risk of predation for males. Moreover, Maya and Marañon (2001) under experimental conditions concluded that the highest temperature alters the sex ratios, being 31°C the one that induced most proportion of males in the native population of P. reticulata. In this study, monthly variations in the sex ratios could be explained too by the effect of the mesh size and the shallowness of the lake, which make difficult the fish capture.
Respect to the relationship between length and weight, Ricker (1975) mentioned that when the fishes have a coefficient b value significantly different from 3, growth is allometric. This is the case of the present study and therefore, it suggests that body parts do not grow at a similar rate and then the fish grow in length more than in weight. Similar results have been found for others species such as P. sphenops (Martinez-Trujillo 1983), P. gracilis (Contreras-MacBeath and Ramírez-Espinoza 1996), Heterandria bimaculata (Gómez-Márquez et al. 1999) and, Poecilia reticulata (Urriola Hernández et al. 2004a).
The porthole livebearer reaches sexual maturity at a small size within its first year of life and is therefore able to begin to reproduce in October. Urriola et al. (2004a) found similar results of sexual maturity for P. reticulata. The reproductive cycle analysis showed that this type of activity is present throughout the year. With the gonadosomatic index and development stages together, it is evident that peak reproduction occurs from July to October, during the rainy season. Contreras-MacBeath and Ramírez-Espinoza (1996) cited similar information for the same species in Morelos State, but at a different location. Burns (1985) found that when P. gracilis was subject to long photoperiods, gonadosomatic index was highest and therefore, reproduction occurred during summer and autumn months, when photoperiod is longer.
The maximum condition value occurred in October coincides with rainy season when the reproductive period was highest. Condition then diminished to December during a reduction in the water temperature. Moreover, is possible than the combination of a lower condition and a greater reproductive effort resulted in a reduction of the individuals as is observed during this period. Furthermore, the decrease in condition has been ascribed to a depletion of body reserves during gonad maturation.
The relationship between fecundity with body size does not demonstrate a significant correlation. Burns (1985) and Contreras-MacBeath and Ramírez-Espinoza (1996) found similar results for superfetating poeciliids, in which an increment in size is not necessarily associated with an increased embryos number. Reznick and Miles (1989) mentioned that in non-superfetating poeciliids there is an association between body size and fecundity as in Poecilia reticulata (Thibault and Schultz 1977), P. turrubarensis (Cabrera and Solano, 1995), Heterandria bimaculata (Gómez-Márquez et al. 1999) and, P. reticulata (Urriola Hernández et al. 2004b). Miller (1975; cited in Burnes, 1985) point out that superfetation is common in Poeciliopsis and therefore, the embryos included in the fecundity estimate for a given female were often in different development stages. In this study, 25 percent of females had several broods in the same reproductive season, which means that porthole livebearer is one of the poeciliids that exhibit superfetation. Likewise, Burns (1985) cited that for P. gracilis, approximately 25 percent of the females in each group showed superfetation and no significant correlation between standard length and egg/ embryos number was seen for each group. In addition, Bagenal (1978) cited that fish species exhibit wide fluctuations in fecundity among fish of the same species, size and age.
Probably, the reproductive cycle in the lake was primarily determinate by the temperature, the seasonal fluctuations of photoperiod, the rainy season (when the water is not totally transparent and the productivity is high) and, the increased water level between July and September, when nutrients for development are available, which affect the timing of the spawning season. Schoenherr (1977) for P. occidentalis, Contreras-MacBeath and Ramírez-Espinoza (1996) for P. gracilis and, vargas and de Sostoa (1996) for Gambusia holbrooki, obtained similar results.
Schoenherr (1977) cited that temperature and day length both influence reproduction in P. occidentalis and that probably is true that many parameters exert an influence, with one or other dominating in different populations or under differing circumstances. Perhaps, water temperature does not vary and therefore, the fishes are responding to an increase in photoperiod and that both factors show their influence not only in terms of the proportion of fishes that carry broods, but also in brood size.
Moreover, Burns (1985) mentioned that slight photoperiod differences could affect the reproductive capabilities of native fish from tropical latitudes. Seasonal day length variations have been implicated in affecting the observed annual fluctuations in brood size in a number of poeciliids species studied (for example, Poeciliopsis gracilis and Poecilia sphenops) in their natural environments, because each population may be able to express different life history traits resulting in adaptations to a particular environment.
The breeding activity for most of poeciliid species increases during periods of intense sunshine and/or rainfall. In this study, a major breeding peak of porthole livebearer is associated with warm temperature, high rainfall and water level rising. The period with high values of GSI for the fish is also the time of increase in phytoplankton biomass (chlorophyll a) at Coatetelco Lake. Phytoplankton biomass in this aquatic system increases following high nutrient concentrations resulting from water mixing and rainfall associated changes in the lakes hydrology. This increase in phytoplankton biomass may play as one of the environmental cues indicating an approaching season favorable for better growth and offspring survival, while the other environmental factors may have indirect effects. A similar conclusion can be drawn also for O. niloticus in Lake Awassa studied by Admassu (1996) and, for O. niloticus from Emiliano Zapata dam (Peña-Mendoza et al., 2005). Nevertheless, a more detailed research is required to investigate the effect of phytoplankton at the time of breeding of P. gracilis in the lake or in other tropical aquatic systems.
The information obtained suggests that a combination of life history traits, early maturity, fast growth and reduced longevity enable porthole livebearer to inhabit different areas and exploit favorable environmental conditions, as in Morelos State and other places. This explains why the Poeciliidae family is the most cultivated at the fish farms of the state mainly with Poecilia reticulata and P. sphenops as the most representative species.
Acknowlegments
We thank everyone who kindly assisted in several parts of the work for collecting, processing and providing measurements of P. gracilis. Our most sincere gratitude to Leticia Huidobro (UNAM, Mexico) and to an anonymous reviewer. This work was supported by the Facultad de Estudios Superiores Zaragoza and the PAPIIT-DGAPA program (project code IN201105-3) of the Universidad Nacional Autónoma de México.
Resumen
Se realizó el análisis reproductivo de 1 225 organismos de Poeciliopsis gracilis que se obtuvieron de manera mensual en el lago Coatetelco, un cuerpo somero tropical en México. Se hizo evidente un dimorfismo sexual en las características morfológicas de esta especie. Se observó una diferencia en el tamaño corporal entre los sexos al inicio de la reproducción. La proporción sexual fue diferente de uno. Con base en la variación mensual del índice gonadosomático (IGS), hepatosomático (IHS) y los estadios de madurez gonádica, se observó que la época de reproducción de P. gracilis se realiza entre julio y octubre, que coincide con la época de lluvias e incremento de la biomasa del fitoplancton. La talla más grande registrada fue de 50 mm para las hembras y de 43 mm para los machos.
Palabras clave: clorofila "a", índice gonadosomático, índice hepatosomático, Poeciliopsis gracilis, reproducción, proporción sexual, México.
Received 19-VI-2007. Corrected 21-V-2008. Accepted 31-VII-2008.
Reference
Admassu, D. 1996. The breeding season of tilapia, Oreochromis niloticus L. in Lake Awassa (Ethiopian rift valley). Hydrobiologia 337: 77-83. [ Links ]
Anonymous. 1981. Síntesis Geográfica de Morelos. Coordinación General de los Servicios Nacionales de Estadística, Geografía e Informática, S.P.P. D.F., México. [ Links ]
Anonymous. 1998. Anuario Estadístico del Estado de Morelos. Instituto Nacional de Estadística, Geografía e Informática. Aguascalientes, Ags. México. [ Links ]
Bagenal, T.B. 1978. Aspects of Fish Fecundity, p. 75-101. In Shelby D.G. (ed.). Ecology of freshwater fish production. Blackwell Scientific Publications, Oxford, Inglaterra. [ Links ]
Burns, R.J. 1985. The effect of low-latitude photoperiods on the reproduction of female and male Poeciliopsis gracilis and Poecilia sphenops. Copeia 4: 961-965. [ Links ]
Cabrera, J. & Y. Solano. 1995. Fertilidad y fecundidad en Poeciliopsis turrubarensis (Pisces:Poeciliidae). Rev. Biol. Trop. 43: 317-320. [ Links ]
Contreras-McBeath, T. & H. Ramírez-Espinoza. 1996. Some aspects of the reproductive strategy of Poeciliopsis gracilis (Osteichthyes: Poeciliidae) in the Cuautla River, Morelos, Mexico. J. Freshwater. Ecol.11: 327-337. [ Links ]
Galindo-villegas, J. & E. Sosa-Lima. 2002. Gonopodial system review and a new fish record of Poeciliopsis infans (Cyprinodontiformes: Poeciliidae) for Lake Patzcuaro, Michoacan, central Mexico. Rev. Biol. Trop. 50: 1151-1157. [ Links ]
Gómez-Márquez, J.L., J.L. Guzmán-Santiago & A. Olvera-Soto. 1999. Reproducción y crecimiento de Heterandria bimaculata (Cyprynodontiformes: Poeciliidae) en la laguna "El Rodeo", Morelos, México. Rev. Biol. Trop. 47: 581-592. [ Links ]
Gómez-Márquez, J.L. 2002. Estudio limnológico-pesquero del lago de Coatetelco, Morelos, México. PhD. Thesis, Facultad de Ciencias, U.N.A.M., México. [ Links ]
Granados, R.J.G. 1990. El comportamiento del zooplancton en tres ambientes acuáticos epicontinentales del estado de Morelos, México. Master of Science Thesis (Biology), Facultad de Ciencias, U.N.A.M., México. [ Links ]
Huidobro, C.L. 2000. Filogenia del complejo Poeciliopsis gracilis Regan (Pisces:Poeciliidae) y su biogeografía. Master in Science Thesis, Facultad de Ciencias U.N.A.M., México. [ Links ]
King , M. 1995. Fisheries Biology, Assessment and Management. 2ed. Ed. Fishing News Books. Oxford, Inglaterra. [ Links ]
Martínez-Trujillo, M. 1983. Contribución al conocimiento de la biología de Poecilia sphenops valencienes (Pisces:Poeciliidae), en la presa de Zicuirán, Mich. Tesis de Licenciatura, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, México. [ Links ]
Mejía, M.H. 1992. Nuevo registro de Poeciliopsis gracilis (Heckel, 1848) (Pisces: Poeciliidae), para la cuenca del Río Balsas. Universidad: Ciencia y Tecnología. 2: 131-136. [ Links ]
Martínez, D., S.H. Marañón & A. Cárdenas. 2004. Análisis retrospectivo de la piscicultura de ornato en el estado de Morelos. Sociedades Rurales Producción y Ambiente, 5: 69-75. [ Links ]
Maya, P.E. & S.H. Marañón. 2001. Efecto de la temperatura sobre la proporción sexual de Poecilia reticulata Peters, 1859 (Pisces:Peciliidae). Hidrobiológica 11: 157-162. [ Links ]
Maya, P.E., S.H. Marañón & N.I.C. Sánchez. 2006. Análisis de un ciclo de producción en una granja familiar productora de poecílidos en el estado de Morelos. Sociedades Rurales, Producción y Ambiente. 6: 67-82. [ Links ]
Meffe, K.G. & F.F. Snelson, Jr. 1989. An ecological overview of poeciliid fishes, p. 13-31. In Meffe, K.G. & F.F. Snelson Jr. (eds). Ecology and evolution of live-bearing fishes. Prentice Hall, Nueva Jersey, EEUU. [ Links ]
Meffe, K. G. 1989. List of accepted common names of poeciliid fishes, p. 369-371. In Meffe, K.G. & F.F. Snelson Jr. (eds). Ecology and evolution of livebearing fishes. Prentice Hall, Nueva Jersey. [ Links ]
Mendoza, G. 1962. The reproductive cycles of three viviparous teleosts Alloophorus robustus, Goodea luitpoldi y Neophorus diazi. Biol. Bull. 123: 351-365. [ Links ]
Miller, R.R. 1966. Geographic distribution of Central American freshwater fishes. Copeia 4: 773-802. [ Links ]
Miller, R.R., W.L. Winckley & S.M. Norris. 2005. Freshwater fishes of México. The University of Chicago, Chicago, EEUU. [ Links ]
Nikolsky, V.G. 1963. The Ecology of Fishes. Academic, Londres, Inglaterra. [ Links ]
Peña-Mendoza, B., J.L. Gómez-Márquez, I.H. Salgado-Ugarte & D. Ramírez-Noguera. 2005. Reproductive biology of Oreochromis niloticus (Perciformes: Cichlidae) at Emiliano Zapata dam, Morelos, México. Rev. Biol.. Trop. 53: 515-522. [ Links ]
Rauchenberger, M. 1989. Annotated species list on the subfamily Poeciliinae, p. 359-368. In: Meffe, K.G. & F.F. Snelson Jr. (eds). Ecology and evolution of livebearing fishes. Prentice Hall, Nueva Jersey. [ Links ]
Reznick, D. 1983. The structure of guppy life histories, the tradeoff between growth and reproduction. Ecology 64: 862-873. [ Links ]
Reznick, D.N. & D.B. Miles. 1989. A review of life history patterns in poeciliid fishes, p. 125-148. In Meffe, G. K. & F. F. Snelson, Jr. (eds). Ecology and evolution of livebearing fishes (Poeciliidae). Ed. Prentice Hall, Nueva Jersey. [ Links ]
Ricker, E.W. 1975. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Board Canada. 191: 145-157. [ Links ]
Rodriguez, M.C. 1997. Phylogenetic analysis of the Tribe Poeciliini (Cyprinodontiformes: Poeciliidae). Copeia, 4: 663-679. [ Links ]
Rosen, E.D. & R.M. Bailey. 1963. The poeciliid fishes (Cyprinodontiformes), their structure, zoogeography and systematics. Bull. Am. Muss. Nat. Hist.126: 5-176. [ Links ]
Rosenthal, G.G., T.Y.F. Martinez, F.J. García de León & M.J. Ryan. 2001. Shared preferences by predators and females for male ornaments in swordtails. Am. Nat. 158:146–154. [ Links ]
Snelson, F.F. Jr. 1984. Seasonal maturation and growth of males in a natural population of Poecilia latipina. Copeia 1: 252-255. [ Links ]
Snelson, F.F. 1989. Social and environmental control of life history traits in poeciliid fishes, p. 149-161. In Meffe, G.K. & F.F. Snelson, Jr. (eds). Ecology and evolution of livebearing fishes (Poeciliidae). Ed. Prentice Hall, Nueva Jersey. [ Links ]
Schoenherr, A.A. 1977. Density dependent and density independent regulation of reproduction in the gila topminnow, Poeciliopsis occidentalis (Baird and Girard). Ecology 58: 438-444. [ Links ]
Thibault, R.E. & R.J. Schultz. 1977. Reproductive adaptations among viviparous fishes (Cyprinodontiformes: Poeciliidae). Evolution. 32: 320-333. [ Links ]
Urriola, H.M., J.P. Cabrera & M.Q. Protti. 2004a. Composición, crecimiento e índice de condición de una población de Poecilia reticulata (Pisces: Poeciliidae) en un estanque en Heredia, Costa Rica. Rev. Biol. Trop. 52: 57-162. [ Links ]
Urriola, H.M., J.P. Cabrera & M.Q. Protti. 2004b. Fecundidad, fertilidad e índice gonadosomático de Poecilia reticulata (Pisces: Poeciliidae) en Heredia, Costa Rica. Rev. Biol. Trop. 52: 945-950. [ Links ]
Vargas, M.J. & A. de Sosota. 1996. Life history of Gambusia holbrooki (Pisces, Poeciliidae) in the Ebro delta (NE Iberian peninsula). Hydrobiologia 341: 215-224. [ Links ]
Wetzel, R.G. & G.E. Likens. 1991. Limnological analyses. W.B. Saunders Company, Filadelfia. [ Links ]