Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.54 suppl.3 San José Dec. 2006
Status of aspergillosis and sea fan populations in Curaçao ten years after the 1995 Caribbean epizootic
M. M. Nugues1,* & I. Nagelkerken2
1 Center for Marine Science, University of North Carolina Wilmington, 5600 Marvin K. Moss Lane, Wilmington North Carolina 28409, USA.
2 Department of Animal Ecology and Ecophysiology, Faculty of Science, Radboud University Nijmegen, Toernooiveld 1, 6525 ED Nijmegen, The Netherlands.
* Corresponding author: Maggy M. Nugues
Present address: Royal Netherlands Institute for Sea Research (NIOZ), P.O. Box 59, 1790 AB Den Burg, Texel, The Netherlands, Tel: +31 222 369558, Fax: +31 222 319674; mnugues@nioz.nl
Received 06-VI-2006. Corrected 02-X-2006. Accepted 13-X-2006.
Abstract: In 1995, a survey of sea fan corals was conducted in Curaçao during a Caribbean-wide outbreak of the sea fan disease aspergillosis. The survey was repeated in 2005 using the same methodology and identical sites to examine changes in sea fan populations 10 years after the initial epizootic. Necrotic lesions typical of aspergillosis were present on as many sea fans in 2005 as in 1995 (mean ± SE: 52 ± 6 vs 43 ± 10%). The disease also showed no significant variation in virulence (9.6 ± 1.2 vs 8.8 ± 1.0% tissue loss per diseased colony). However, the average number of sea fan colonies per 10 m2 decreased from 2.7 ± 1.1 to 0.7 ± 0.2 over the 10-year period, a decline of almost 75%. This decrease occurred for all colony sizes, but was more pronounced among small colonies, resulting in an overall trend of domination by large colonies. These results support that aspergillosis can have a significant, long-term impact on sea fan population size and structure. The continued presence of the disease in 2005 could be contributing to reduced recruitment and/or selective mortality among the smallest colonies. This study provides no indication that host resistance against aspergillosis could reverse the decline of Caribbean sea fan corals. Rev. Biol. Trop. 54 (Suppl. 3): 153-160. Epub 2007 Jan. 15.
Key words: Aspergillosis, Caribbean, coral disease, coral reefs, gorgonians, sea fan, population structure.
Coral reefs worldwide have experienced unprecedented losses in coral cover in the past 30 years (Wilkinson 2002, Gardner et al. 2003, Hughes et al. 2003). There is widespread concern that coral diseases might be a major cause of this decline (Richardson 1998, Harvell et al. 1999, Dustan 1999, Porter et al. 2001). Over the past three decades, there has been an increase in the number of reports documenting disease outbreaks affecting scleractinian corals (Richardson et al. 1998, Nugues 2002, Patterson et al. 2002, Weil et al. 2002, Borger 2003, Cróquer et al. 2003, Miller et al. 2003, Borger and Steiner 2005) and octocorals (Guzmán and Cortés 1984, Garzón-Ferreira and Zea 1992, Nagelkerken et al. 1997a, b, Weil et al. 2002, Kim and Harvell 2002, 2004), each generally associated with significant mortality. Perhaps the most significant effect of coral disease to date is the virtual elimination of major reef-building acroporids throughout the Caribbean by white band disease (Gladfelter 1982, Aronson and Pretch 1997, 2001). This disease is believed to have initiated shifts in coral dominance towards small, non-frame-work agaricids in at least several Caribbean locations (Aronson and Pretch 1997, 2001).
Despite the potential importance of coral diseases in coral reef degradation, there are few studies quantifying their long-term effects on coral community and population structure. Most studies document the initial impact of epizootics at the most affected locations (Richardson 1998, Harvell et al. 1999, Bruckner 2002). However, it is likely that diseases undergo periodic increases in abundance and virulence at different locations depending on the properties of pathogens, host resistance and environmental conditions (Bruckner 2002, Harvell et al. 2002, Kim and Harvell 2004). Furthermore, any effect of disease can be confounded by a number of other disturbances such as sedimentation, hurricanes or coral bleaching events. To help unravel the role of coral diseases in coral reef decline, it is necessary to quantify spatial and temporal changes in disease characteristics and populations of affected species over several years and a large geographic area.
Aspergillosis is a fungal disease affecting sea fan corals. It was first reported in 1995 in Saba, Netherlands Antilles, and subsequently documented throughout the Caribbean basin during the same year (Nagelkerken et al. 1997a, b). The epizootic was attributed to a fungal soil saprobe belonging to the genus Aspergillus (Smith et al. 1996) and subsequently as A. sydowii (Geiser et al.1998). During the Caribbean-wide 1995 outbreak, aspergillosis affected between 10 and 90% of sea fan colonies (Nagelkerken et al. 1997b), which is among the highest prevalence recorded for any coral disease. Furthermore, virulence (defined as the percentage of tissue loss per diseased colony) varied between 3 and 45% (Nagelkerken et al. 1997b), indicating that the disease can cause widespread tissue mortality in affected colonies.
At present, little is known about the spatial and temporal variability of aspergillosis and its long-term effects on octocoral populations. During the 1995 epizootic, Nagelkerken et al.(1997b) surveyed aspergillosis and sea fan populations at six sites along the southern coast of Curaçao, Netherlands Antilles. In 2005, we repeated the survey using the same methodology and identical sites. This paper examines changes in the prevalence and virulence of the disease, as well as in sea fan abundance and population size structure, between 1995 and 2005.
Materials and methods
Survey sites and methods: Between October and November 1995, Nagelkerken et al. (1997b) surveyed the prevalence and virulence of aspergillosis in sea fans at six sites on the leeward coast of Curaçao, Netherlands Antilles (Fig. 1, for detailed descriptions of all sites see Van Duyl 1985). In March 2005, the disease was resurveyed at the same sites using the same methodology and disease identification signs (Nagelkerken et al. 1997a, b). At each site, sea fans were surveyed at 3 to 10 m depth using 4 m wide belt transects varying in length from 10 to 125 m, depending on the density of colonies (i.e. longer transects were used in areas with low sea fan abundance and vice versa). Sea fans occur primarily in patches on the shallow reef terrace (100 – 200 m wide, and running from 0 to approx. 8 m deep) and on upper fore reef slopes in Curaçao. Therefore, to position each transect, the diver swam until being able to spot several colonies (ca. 3-4). At this point, a small lead weight attached to the start of the transect line was haphazardly dropped and the transect was laid out in a straight line parallel to the shore. Sufficient (2-8) transects were deployed to obtain a minimum sample of 50 colonies per site, excluding one site where sea fan densities were very low (exact sample sizes shown in Fig. 2A). In total, 5300 and 7376 m2 of reef area was surveyed in 1995 and 2005, respectively.
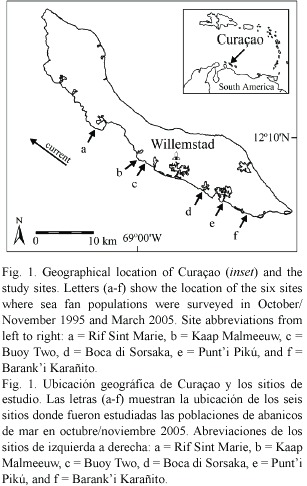
No distinction was made between Gorgonia flabellum (Linnaeus 1758) and G. ventalina (Linnaeus 1758), and both species were combined as Gorgonia spp. Diseased sea fans were identified as those with one or more necrotic patches (i.e. lesions) surrounded by yellow or dark purple coenenchyme (Nagelkerken et al. 1997a, b, Kim and Harvell 2002, 2004). For each diseased colony, we measured the surface area of healthy and diseased tissue using a 50 x 50 cm quadrat divided with strings to create 100 cm2 squares. Maximum colony height was recorded to the nearest cm. Disease prevalence was calculated as the percent of diseased sea fans in the entire population at a site. Virulence was quantified as the percentage of tissue loss (i. e. lesion area) per affected colony. Finally, sea fan density was assessed at each site by dividing the total number of individuals by the total area surveyed.
Statistical analysis: Differences between years in the prevalence and virulence of aspergillosis and density of sea fans were tested with paired t -tests with sites as replicates. Differences among sites in the prevalence of aspergillosis were analysed using a heterogeneity G -test for each year, followed by a simultaneous test procedure (STP) to determine homogeneous groups (Sokal and Rohlf 1995). Site differences in the virulence of aspergillosis were analysed with the Kruskal-Wallis test, followed by post-hoc comparisons using Games-Howell tests when appropriate. No statistical test could be conducted on differences between sites in the density of sea fans because there was no replication within each site (data were not recorded per transect, but pooled within each site). Differences between years in the size (height) frequency distributions were tested, for each site, with Kolmogorov-Smirnov two-sample tests (Sokal and Rohlf 1995).
Results
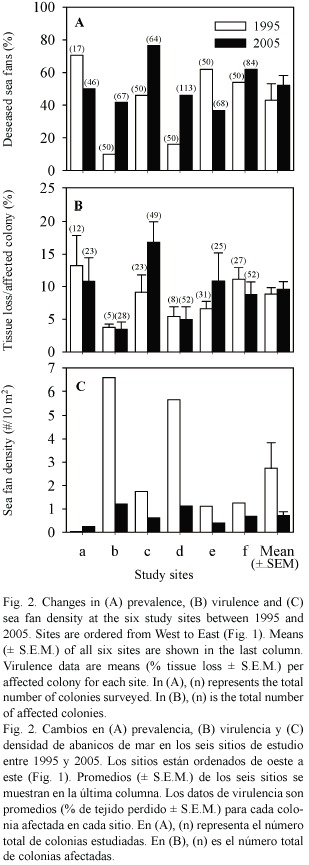
Aspergillosis prevalence and virulence: Comparison between the 1995 and 2005 surveys showed no significant variation in prevalence (mean ± SE: 43.1 ± 10.1 vs 52.2 ± 6.0%) or virulence (8.8 ± 1.0 vs 9.6 ± 1.2%) of aspergillosis between years (paired t -tests: prevalence: t = -0.843, df = 5, p = 0.438, virulence: t = -0.632, df = 5, p = 0.555, Fig. 2A & B). Disease prevalence varied significantly among sites in both years (heterogeneity G - test: 1995: GH= 57.26, df = 5, p < 0.001, 2005: GH= 30.60, df = 5, p < 0.001), with values ranging from 10 to 71% in 1995 and 37 to 77% in 2005. STP tests indicated two homogeneous site groups in 1995 (b, d and a, c, e, f) and three groups in 2005 (a, b, d, e and a, b, d, f and c, f). Virulence varied significantly among sites in 2005 (Kruskal-Wallis test: 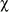
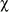
Sea fan population size and structure: The average number of sea fan colonies per 10 m2 decreased from 2.74 ± 1.10 in 1995 to 0.72 ± 0.25 in 2005 (Fig. 2C). Although marginally non-significant (paired t-test: t = 2.212, df = 5, p = 0.087), this decrease represents a decline of 74% in sea fan population size over the past decade. When pooling all sites, this decline affected all height classes of sea fans, but there was a more pronounced decline in the number of small-sized colonies, especially those between 11 and 30 cm in height (Fig. 3).When summing the two smallest size classes (sea fans 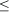
The prevalence of disease increased with sea fan size in both years (Fig. 4A), whereas virulence showed a slight decline with size (Fig. 4B). Interestingly, the prevalence of disease in smaller sized sea fans was significantly higher in 2005 than in 1995 (Chi-square test using years and the three smallest size classes as variables, 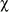
Discussion
The prevalence and virulence of aspergillosis remained unchanged in Curaçao between 1995 and 2005. Since no data were collected in the period between 1995 and 2005, it is unknown whether the disease has been continuously present and exhibited fluctuations in prevalence and virulence. However, the duration of aspergillosis may be similar to white band disease (Kim and Harvell 2004). In the Florida Keys, aspergillosis was present every year between 1997 and 2003, and, although showing anoverall decline in prevalence, it exhibited temporary periods of reemergence at several sites (Kim and Harvell 2004). Our study provides further support for the long duration of this disease.
Curaçaos sea fan population experienced an almost 75% decline in abundance between 1995 and 2005. This decrease, together with the continued presence of the disease in 2005, suggests that aspergillosis could have a significant regulatory impact on sea fan populations. Aspergillosis is characterized by the presence of large lesions on affected sea fan colonies (Nagelkerken et al. 1997a, b, Kim and Harvell 2002, 2004). Such lesions can lead to a reduction in colony size and/or complete colony mortality, affecting sea fan abundance and size-frequency distribution. In this study, while the decline in sea fan abundance affected all colony sizes, it was more pronounced in small colonies. Such demographic shift suggests either a decrease in recruitment, or disproportionably high mortality of the smallest colonies.
Many factors could contribute to such demographic shift. Sea fans are affected by multiple stressors, including breakage from hurricanes (Yoshioka and Yoshioka 1987), overgrowth by fouling organisms (Yoshioka and Yoshioka 1991), grazing by snails or fishes (Lasker 1985, Harvell and Suchanek 1987, Nagelkerken et al. 1997b), and predation by Hermodice carunculata (Pallas, 1766) (Wahle 1985, Vreeland and Lasker 1989). However, there is little indication that any of these factors has increased over the past decade and could selectively affect sea fan reproduction or survivorship of small colonies.
In contrast, aspergillosis has recently been shown to affect reproduction in infected sea fans (Petes et al. 2003). As larger sea fans produce far more gametes than smaller colonies (Beiring and Lasker 2000), the particularly high prevalence of the disease in larger sea fans (this study, Nagelkerken et al. 1997b, Kim and Harvell 2002, 2004), coupled with their decline in abundance, could lead to a rapid decrease in larval supply and subsequent recruitment. Alternatively, recruitment may occur, but consist of colonies susceptible to the disease leading to high mortality among the smallest sea fans. This scenario is supported by the higher disease prevalence among small colonies in 2005 compared to 1995, and a higher proportion of live tissue loss in small compared to large colonies, but is inconsistent with the episodic recruitment events of sea fans with low disease prevalence reported in the Florida Keys (Kim and Harvell 2004).These events offset the loss of large colonies caused by the disease and led to relatively unchanged sea fan densities, as well as reduced disease prevalence, in the Florida Keys between 1997 and 2003.
The reason why successful recruitment occurred in the Florida Keys, and not in Curaçao, is unclear, but could include differences in the environment, pathogen input and/ or virulence, and/or host resistance. At present, there is no indication that environmental conditions, such as pathogen input or temperature, may differ between these two locations. Dube et al. (2002) reported a lower variance in anti-fungal activity at reefs with greater disease intensity, suggesting that selection for aspergillosis resistance could occur over time. Unlike the Florida Keys, the decline in sea fan abundance in Curaçao provides no evidence for such selection, and is a cause for concern regarding the fate and recovery of Caribbean sea fan corals.
Acknowledgments
We thank the staff of the CARMABI research institute - especially W. Bakhuis, A. Debrot, L. Pors, B. Leysner and C. Winterdaal, the staff of the Office of International Program at UNCW, and A. Szmant for their support of the project. We are very grateful to the students of the UNCW Spring 2005 BIO585 Coral Reef Field Course, namely A. Bright, T. Cyronak, K. Johnson, J. McKenzie, K. Neely, M. Ovard, V. Schrameyer, C. Wagner, A. Watson, J. Wilkie and M. Williams for assistance with field work. This research was funded by the Island Government of Curaçao and the central government of the Netherlands Antilles, by their annual subsidy to the Carmabi Foundation in 1995 and by the Coral Reef Research programme at UNCW in 2005.
Resumen
En 1995, se realizó un sondeo de los abanicos de mar durante un brote de aspergilosis, una enfermedad de abanicos de mar extendida en todo el Caribe. En el año 2005 se repitió el sondeo utilizando exactamente la misma metodología y los mismos sitios para examinar cambios en las poblaciones tras 10 años del inicio del brote. Se presentaron lesiones necróticas típicas de la aspergilosis en tantos abanicos en el 2005, como en 1995 (promedio ± ES: 52 ± 6 vs 43 ± 10%). La enfermedad tampoco mostró variaciones significativas en la virulencia (9.6 ± 1.2 vs 8.8 ± 1.0%, pérdida de tejido por colonia enferma). Sin embargo, el número promedio de colonias de abanico de mar por cada 10 m2 bajó desde 2.7 ± 1 hasta 0.7 ± 0.2 en este período de 10 años, una disminución de casi 75%. Este decrecimiento ocurrió en colonias de todo tamaño, pero fue más pronunciado en colonias pequeñas, produciendo una tendencia general de dominancia de colonias grandes. Estos resultados apoyan la idea de que la aspergilosis puede tener un impacto significativo a largo plazo en el tamaño y estructura poblacional de los abanicos de mar. La continuidad en la presencia de la enfermedad en el 2005 puede estar contribuyendo a la reducción en el reclutamiento y/o a la mortalidad selectiva de las colonias más pequeñas. Este estudio no provee ninguna evidencia de que la resistencia del hospedero contra la aspergilosis pueda revertir el decrecimiento de los abanicos de mar en el Caribe.
Palabras clave: aspergilosis, Caribe, enfermedad de coral, arrecife de coral, gorgonáceos, abanico de mar, estructura poblacional.
References
Aronson, R. B. & W. F Precht. 1997. Stasis, biological disturbance, and community structure of a Holocene coral reef. Paleobiol. 23: 326-346. [ Links ]
Aronson, R. B. & W. F Precht. 2001. White-band disease and the changing face of Caribbean coral reefs. Hydrobiol. 460: 25-38. [ Links ]
Beiring, E. A. & H. R. Lasker. 2000. Egg production by colonies of a gorgonian coral. Mar. Ecol. Prog. Ser. 196: 169-177. [ Links ]
Borger, J. L. 2003. Three scleractinian coral diseases in Dominica, West Indies: Distribution, infection patterns and contribution to coral tissue mortality. Rev. Biol. Trop. 51 (Suppl. 4): 25-38. [ Links ]
Borger, J. L. & S. C. C. Steiner. 2005. The spatial and temporal dynamics of coral diseases in Dominica, West Indies. Bull. Mar. Sci. 77: 137-154. [ Links ]
Bruckner, A. 2002. Priorities for effective management of coral diseases. NOAA technical memorandum NMFS-OPR-22. US Dept Commerce. 54 p. [ Links ]
Cróquer, A., S. M. Pauls & A. L. Zubillaga. 2003. White plague disease outbreak in a coral reef at Los Roques National Park, Venezuela. Rev. Biol. Trop. 51 (Suppl. 4): 39-45. [ Links ]
Dube, D., K. Kim, A. P. Alker & C. D. Harvell. 2002. Size structure and geographic variation in chemical resistance of sea fan corals Gorgonia ventalina to a fungal pathogen. Mar. Ecol. Prog. Ser. 231: 139-150. [ Links ]
Dustan, P. 1999. Coral reefs under stress: sources of mortality in the Florida Keys. Nat. Res. Forum 23: 147-155. [ Links ]
Gardner, T. A., I. M. Côté, J. A. Gill, A. Grant & A. R. Watkinson. 2003. Long-term region-wide decline in Caribbean corals. Science 301: 958-960. [ Links ]
Garzón-Ferreira, J. & S. Zea. 1992. A mass mortality of Gorgonia ventalina (Cnidaria: Gorgoniidae) in the Santa Marta area, Caribbean coast of Colombia. Bull. Mar. Sci. 50: 522-526. [ Links ]
Geiser, D. M., J. W Taylor, K. B. Ritchie & G. W. Smith. 1998. Cause of sea fan death in the West Indies. Nature 394: 137-138. [ Links ]
Gladfelter, W. 1982. Whiteband disease in Acropora palmata: implications for the structure and growth of shallow reefs. Bull. Mar. Sci. 32: 639-643. [ Links ]
Guzmán, H. M.& J. Cortés. 1984. Mass death of Gorgonia flabellum L. (Octocorallia: Gorgonidae) on the Caribbean coast of Costa Rica. Rev. Biol. Trop. 32: 305-308. [ Links ]
Harvell, C. D. & T. H. Suchanek. 1987. Partial predation on tropical gorgonians by Cyphoma gibbosum (Gastropoda). Mar. Ecol. Prog. Ser. 38: 37-44. [ Links ]
Harvell, C. D., K. Kim, J. M. Burkholder, R. R. Colwell, P. R. Epstein, D. J. Grimes, E. E. Hofmann, E. K. Lipp, A. D. M. E. Osterhaus, R. M. Overstreet, J. W. Porter, G. W. Smith & G. R. Vasta. 1999. Emerging marine diseases - climate links and anthropogenic factors. Science 285: 1505-1510. [ Links ]
Harvell, C. D., C. E. Mitchell, J. R. Ward, S. Altizer, A. Dobson, R. S. Ostfeld & M. D. Samuel. 2002. Climate warming and disease risks for terrestrial and marine biota. Science 296: 2158-2162. [ Links ]
Hughes, T. P , A. H. Baird, D. R. Bellwood, M. Card, S. R. Connolly, C. Folke, R. Grosberg, O. Hoegh-Guldberg, J. B. C. Jackson, J. Kleypas, J. M. Lough, P. Marshall, M. Nyström, S. R. Palumbi, J. M. Pandolfi, B. Rosen & J. Roughgarden. 2003. Climate change, human impacts, and the resilience of coral reefs. Science 301: 929-933. [ Links ]
Kim, K. & C. D. Harvell. 2002. Aspergillosis in sea fan corals: disease dynamics in the Florida Keys, p. 813-824. In J. W. Porter & K. G. Porter (eds.).The Everglades, Florida Bay, and Coral Reefs of the Florida Keys: An Ecosystem Sourcebook. CRC Press, Boca Raton, Florida. [ Links ]
Kim, K. & C. D. Harvell. 2004. The rise and fall of a six-year coral-fungal epizootic. Amer. Nat. 164: S52-S63. [ Links ]
Lasker, R. H. 1985. Prey preferences and browsing pressure of the butterflyfish Chaetodon capistratus on Caribbean gorgonians. Mar. Ecol. Prog. Ser. 21: 213-220. [ Links ]
Miller, J., C. Rogers & R. Waara. 2003. Monitoring the coral disease, plague type II, on coral reefs in St. John, U. S. Virgin Islands. Rev. Biol. Trop. 51 (Suppl. 4):47-55. [ Links ]
Nagelkerken, I., K. Buchan, G. W. Smith, K. Bonair, P. Bush, J. Garzón-Ferreira, L. Botero, P. Gayle, C. Heberer, C. Petrovic, L. Pors & P. Yoshioka. 1997a. Widespread disease in Caribbean sea fans: I. Spreading and general characteristics. Proc. 8th Int. Coral Reef Symp., Panama 1: 679-682. [ Links ]
Nagelkerken, I., K. Buchan, G. W. Smith, K. Bonair, P. Bush, J. Garzón-Ferreira, L. Botero, P. Gayle, C. D. Harvell, C. Heberer, K. Kim, C. Petrovic, L. Pors & P. Yoshioka. 1997b. Widespread disease in Caribbean sea fans: II. Patterns of infection and tissue loss. Mar. Ecol. Prog. Ser. 160: 255-263. [ Links ]
Nugues, M. M. 2002. Impact of a coral disease outbreak on coral communities in St. Lucia: what and how much has been lost? Mar. Ecol. Prog. Ser. 229: 61-71. [ Links ]
Patterson, K. L., J. W. Porter, K. B. Ritchie, S. W. Polson, E. Mueller, E. C. Peters, D. L. Santavy & G. W. Smith. 2002. The etiology of white pox, a lethal disease of the Carribean elkhorn coral, Acropora palmata. Proc. Natl. Acad. Sci. USA 99: 8725- 8730. [ Links ]
Petes, L. E., C. D. Harvell, E. C. Peters, M. A. H. Webb & K. M. Mullen. 2003. Pathogens compromise reproduction and induce melanization in Caribbean sea fans. Mar. Ecol. Prog. Ser. 264: 167-171 [ Links ]
Porter, J. W., P. Dustan, W. C. Jaap, K. L. Patterson, V. Kosmynin, O. W. Meier, M. E. Patterson & M. Parsons. 2001. Patterns of spread of coral disease in the Florida Keys. Hydrobiol. 460: 1-24. [ Links ]
Richardson, L. L. 1998. Coral diseases: what is really known? Trends Ecol. Evol. 13: 438-443. [ Links ]
Richardson, L. L., W. M. Goldberg, K. G. Kuta, R. B. Aronson, G. W. Smith, K. B. Ritchie, J. C. Halas, J. S. Feingold & S. L. Miller. 1998. Floridas mystery coral-killer identified. Nature 392:557-558. [ Links ]
Smith, G. W., L. D. Ives, I. Nagelkerken & K. B. Ritchie. 1996. Caribbean sea-fan mortalities. Nature 383: 487. [ Links ]
Sokal, R. R. & F. J. Rohlf. 1995. Biometry, 3rd ed. Freeman, New York. 887 p. [ Links ]
Van Duyl, F. C. 1985. Atlas of the living reefs of Curaçao and Bonaire, Netherlands Antilles. Publ. Found. Sci. Res. Caribb. Reg. 117: 1-13. [ Links ]
Vreeland, A. L. & H. R. Lasker. 1989. Selective feeding of the polychaete H. carunculata (Pallas) on Caribbean gorgonians. J. Exp. Mar. Biol. Ecol. 129: 265-277. [ Links ]
Wahle, C. M. 1985. Habitat-related patterns of injury and mortality among Jamaican gorgonians. Bull. Mar. Sci. 37: 905-927. [ Links ]
Weil, E., I. Urreiztieta & J. Garzón-Ferreira. 2002. Geographic variability in the incidence of coral and octocoral diseases in the wider Caribbean. Proc. 9th Int. Coral Reef Symp., Bali 2: 1231-1238. [ Links ]
Wilkinson, C. 2002. Status of Coral Reefs of the World: 2002. Austr. Inst. Mar. Sci., Townsville. 378 p. [ Links ]
Yoshioka, P. M. & B. B. Yoshioka. 1987. Variable effects of hurricane David on the shallow water gorgonians of Puerto Rico. Bull. Mar. Sci. 40: 132-144. [ Links ]
Yoshioka, P. M. & B. B. Yoshioka. 1991. A comparison of the survivorship and growth of shallow-water gorgonian species of Puerto Rico. Mar. Ecol. Prog. Ser. 69: 253-260. [ Links ]














