Revista de Biología Tropical
versión On-line ISSN 0034-7744versión impresa ISSN 0034-7744
Rev. biol. trop vol.54 supl.3 San José dic. 2006
The recent decline of Montastraea annularis (complex) coral populations in western Curaçao: a cause for concern?
A. W. Bruckner & R. J. Bruckner
NOAA Fisheries, Coral Reef Conservation Program, Office of Habitat Conservation, 1315 East West Highway, Silver Spring, MD 20910, USA; andy.bruckner@noaa.gov
Received 01-VI-2006. Corrected 02-X-2006. Accepted 13-X-2006.
Abstract: Shallow leeward reefs off the western end of Curaçao are dominated by extensive populations of M. annularis (complex). These species are larger in size (mean= 66 cm diameter) than all other species, with few small colonies (<30 cm) and notable absence of recruits. In 1998, colonies of M. annularis (complex) accounted for more then 45% of all species >10 cm observed within transects, and most exhibited low levels of partial mortality (mean= 22.5%). These species were less abundant (38% of all colonies) in 2005. Partial mortality among live colonies of M. annularis and M. faveolata increased by 85% (mean = 42% partial mortality) and numerous dead colonies of M. faveolata and M. annularis were observed; M. franksi colonies were generally in excellent condition (14% partial tissue mortality). A high prevalence of coral diseases (3-30%) was documented among M. annularis and M. faveolata, while all other species were less frequently affected. Yellow band disease (YBD) emerged shortly after the 1995 bleaching event, and rapidly spread throughout all depths, with the highest prevalence between 1997-1999. YBD caused slow rates of mortality (=1 cm/month), but multiple focal lesions appeared on individual colonies, and these progressively radiated outward as they killed the colonies. By 2005, 44% of the tagged corals were dead; the remainder exhibited active YBD infections (21%) or were in remission (31.6%) but were missing on average >90% of their tissue. Although the incidence of YBD has declined since 2000, white plague (WP) prevalence was unusually high (4-12%) in 2001 and 2005, with affected colonies exhibiting recent mortality of up to 70%. Dead Montastraea spp. surfaces are being colonized by other corals, including poritids, agaricids, and other faviids, while recruits of M. annularis (complex) are absent. If diseases and other biotic stressors persist on these reefs, M. annularis and M. faveolata populations may undergo a decline similar to that observed in the 1980s among Caribbean acroporids. Rev. Biol. Trop. 54 (Suppl. 3): 45- 58. Epub 2007 Jan. 15.
Key words: coral monitoring, coral disease, yellow-band disease, white plague, star coral, Montastraea annularis (complex), Curaçao, Caribbean.
Until recently, shallow Caribbean reefs were dominated by three common scleractinian corals, Acropora palmata, A. cervicornis and Montastraea annularis (Goreau 1959, Goreau and Goreau 1973). Acropora palmata was found primarily in the reef crest and shallow fore reef from 0-5 m depth, were it formed extensive monospecific thickets (the palmata zone ). Acropora cervicornis was dominant at intermediate depths (5-15 m) on exposed reefs (the cervicornis zone) and in shallower habitats on more protected reefs (1-10 m depth), often coexisting with M. annularis (Adey and Burke 1976, Geister 1977, Van Duyl 1985). The sibling species of Montastraea (M. annularis, M. faveolata and M. franksi; Knowlton et al. 1992, Weil and Knowlton 1994) had the widest distribution, occurring in both protected and wave-exposed habitats from just below the surface of the water to over 80 m depth (Goreau and Wells 1967, Van Duyl 1985).
Montastraea annularis (complex) historically ranked in importance with A. palmata and A. cervicornis in its overall contribution to Western Atlantic reef structure (Jackson 1992). The abundance of acroporids has been reduced by 80-98% throughout vast portions of their range over the last several decades, and M. annularis (complex) may now represent the most important framework coral in the western Atlantic (Goreau 1959, Glynn 1973, Liddel and Ohlhorst 1986, Aronson and Precht 2001, Bruckner 2003). Montastraea spp. are often numerically dominant at shallow and intermediate depths, and they constitute a significant portion of the total living coral cover in many locations. Although Montastraea spp. colonies exhibit much slower growth rates than the Acropora species (e. g., 1-15 mm/year; Dustan 1975, Weber and White 1977, Highsmith et al. 1983, Hudson 1981), colonies are much longer lived, and can form massive structures several meters in height and diameter. In addition to their contribution to reef growth, colonies of M. annularis (complex) provide high structural relief used by numerous associated coral reef species as nursery, resting, feeding and spawning habitat (Dustan 1975).
The high densities of large colonies of M. annularis (complex) observed throughout the Caribbean suggests that, at least until recently, these species were less susceptible to the stressors that contributed to the demise of other species, including physical disturbance, bleaching, disease and predation (Bythell et al. 1993). These corals are extremely robust and resist the effects of all but the most severe storms and hurricanes (Stoddart 1963, Woodley et al. 2003, Bythell et al. 1993). Of the three species, columnar lobate M. annularis colonies are most frequently fragmented during storms, but when damaged these corals exhibit high rates of healing and survival when compared to other corals (Bak and Engel 1979). These species are also particularly susceptible to predation by Sparisoma viride, although all but the largest of the injuries appear to rapidly heal in absence of compounding stressors (Bruckner and Bruckner 2002). Furthermore, the increase in macroalgae observed on many reefs following the die-off of Diadema appears to have had minimal effects on the larger colonies of Montastraea spp.
Environmental conditions appear to have degraded throughout the Caribbean since the late 1990s, as evidenced by an emergence of several particularly virulent diseases, recent bleaching events and other biotic disturbances, and increasing rates of mortality among species previously thought to be resistant to these stressors (Richardson 1998, Green and Bruckner 2000, Sutherland et al. 2004, Weil 2004).The sibling species of Montastraea are among the most susceptible species, with colonies affected by up to eight different diseases (Weil 2004). Yellow-band disease has become particularly insidious, with outbreaks affecting 18-91% of the Montastraea colonies in reefs examined in Panama, the Netherland Antilles, Colombia, Mexico, Puerto Rico, Venezuela and other locations (Santavy et al. 1999, Cervino et al. 2001, Bruckner and Bruckner 2003, Gil-Agudelo et al. 2004, Jordán-Dahlgren and Rodríguez-Martínez 2004, Bruckner and Bruckner in press). Yellow band disease was first observed in Curaçao in 1995 (Paul Hoetjes pers. comm. 1998); quantitative surveys by Bruckner and Bruckner (2003) report that up to 49% of M. annularis colonies were affected in 1997 and 68% in 1998. In addition, recent outbreaks of a condition resembling white plague that causes tissue loss at rates of up to decimeters per day (Richardson and Aronson 2002) first occurred in Curaçao during the year following Hurricane Lenny (Hoetjes 2003), and has continued to affected corals in recent years (Nugues et al. 2005, Bruckner unpubl. obs.). Given the importance of these corals as the primary framework builders on reefs today, the potential decline of these species from disease is a cause for concern (Kojis and Quinn 1993). M. annularis (complex) have life spans of decades to centuries and they exhibit slow rate of growth and infrequent, episodic recruitment, suggesting that several decades or more may be required for their replacement (Szmant 1991).
The purpose of this study was to characterize the effects of coral diseases and other biotic stressors on populations of M. annularis (complex) in Curaçao. We evaluated the prevalence of coral diseases, extent of mortality from diseases, and ability of Montastraea annularis (complex) populations to sustain chronic partial mortality through regrowth and recruitment over an eight year period. The Atlantic and Gulf Rapid Reef Assessment (AGRRA) protocol was used to assess the composition, size and condition of reef-building corals, and characterize patterns and causes of mortality. Individual colonies identified with signs of disease were also tagged and evaluated over time to determine the extent of mortality, patterns of colonization of exposed skeletal substratum, and the potential for these communities to recover through regrowth and recruitment.
Materials and methods
This study combines belt transects and of individual tagged colonies between 1997-2005 to assess the prevalence of diseases, extent of partial and total colony mortality, and effects of disease on colony survival. Random transects (30 m long x 1 m wide) were extended along five depths (5, 10, 15, 20 and 25 m depth) on six reefs in Western Curaçao to determine the prevalence of coral diseases and other potential sources of mortality. Permanent transects were also established on leeward reefs at the western end of Curaçao (Lagun, Jeremi, and Kalki) to evaluate hanges in coral species composition, size structure, and condition in more detail (Fig. 1). On these three reefs, the species composition, coral size (maximum diameter and height measured to the nearest cm), condition(percent recent dead and percent long dead visually estimated from a planar view) and source of mortality (disease, predation or overgrowth by algae or invertebrates) for colonies 10 cm in diameter or larger was assessed in 1998, 2000 and 2005 using a modified AGGRA approach (Kramer and Lang 2003). Comparative surveys of composition, size structure, and condition were conducted at least one time on the other three reefs.
Recent mortality was defined as any tissue loss occurring within approximately the last 30 days, and signs included: (1) white coral skeleton that lacked algae (surfaces denuded of tissue within the last 5-7 days); (2) skeletal areas with readily recognizable corallites that had not been substantially eroded but were colonized by green filamentous algae; or (3) white, exposed skeletal surfaces, or eroded skeletal surfaces with fine filamentous algae, that had been physically abraded by fish or other agents but had not yet been colonized by macroalgae, coralline algae or invertebrates. Causes of recent mortality were identified as disease [e.g., black band disease (BBD), white plague (WP), yellow band disease (YBD), white band disease (WBD), dark spots disease (DSD), or other disease), corallivory [focused and spot biting by parrotfish, damselfish (Stegastes planifrons) algal lawns, or snail predation], overgrowth by algae or an invertebrate (cnidarian, sponge or tunicate), physical damage (e.g., storms), or were recorded as unknown.
A minimum of 25 colonies with signs of YBD were tagged on each of three reefs in Curaçao (Jeremi, Lagun and Kalki; n= 100 total) in 1997 and 1998. These were reexamined periodically over the last seven years to determine the duration of infections, overall amount of tissue loss, extent of regrowth, and patterns of colonization of denuded skeletal surfaces by coral recruits or other organisms.
For statistical analyses the three species of M. annularis complex were pooled and compared corals to all other species, and comparisons were also made among the three sibling species of Montastraea for different reefs, depths and years. Log-transformation for length measurements and arc-sine transformation for percentages were used as necessary prior to analyses to normalize data. A students t -test was used for examination of the M. annularis complex versus all other species pooled, or ANOVA (single-factor or two-factor) for comparisons among species, locations, and depth, and differences in coral composition, size frequency distribution and percent partial mortality between reefs and years. When relevant, post-hoc analyses were performed using a Tukey HSD multiple comparison test.
Results
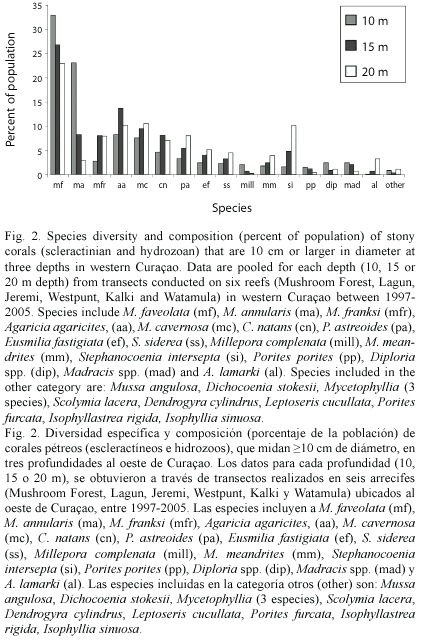
Species composition and size frequency: Leeward reefs examined from 5-25 m depth had a maximum of 29 species of reef building corals (>10 cm in diameter) within a single transect. The three sibling species of Montastraea were more abundant than all other taxa, and collectively made up 30-70% of all corals observed within transects. Other dominant species were Agaricia agaricites, M. cavernosa, Colpophyllia natans, Stephanocoenia intersepta, Porites astreoides, Eusmilia fastigiata, Siderastrea siderea, and Meandrina meandrites. Eighteen additional species were present at low abundances. Formerly dominant species (Acropora cervicornis) were absent, even though large areas of A. cervicornis rubble were present in shallow (10 m depth) areas. Most of the common corals were equally abundant at all depths, except for M. franksi, A. lamarki, S. intersepta which were most common at 20 m depth, and M. annularis which had the highest abundance at 10 m depth (Fig. 2). At all depths and on all reefs, the three sibling species of Montastraea were collectively more abundant than all other species >10 cm diameter in 1998, and together were 46% of all colonies recorded along transects. These species were still the most important corals on these reefs in 2005, although they declined in overall abundance to 38% of the total coral population. Some of these changes can be attributed to the presence of a large number of standing dead M. annularis and M. faveolata colonies observed in 2005 along with an increase in brooding corals (Porites and Agaricia ) that colonized dead M. annularis (complex) surfaces.
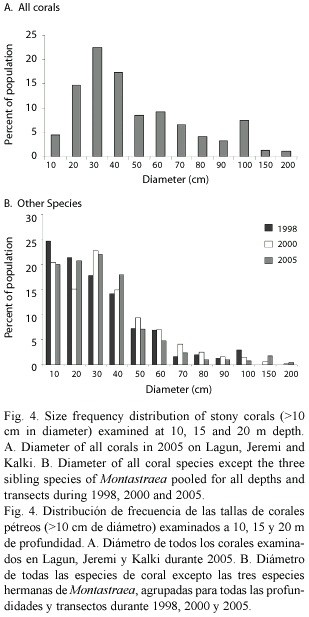
Between 1998-2005 there was no significant shift in the size (diameter) of living corals found on the three western reefs for all species pooled (mean= 49 cm in 1998 versus 52 cm in 2005). Colonies of M. annularis (complex) were larger in size in 1998 (mean= 62 cm diameter) than all other species (mean=43 cm), with less than 10% of all M. annularis (complex) colonies under 30 cm in diameter (Table 1; Fig. 3A). Of the three sibling species, M. faveolata was the largest (72 cm), while M. franksi was the smallest (40 cm). Colony size overall for pooled species of Montastraea did not differ significantly between years, although in 2005 a greater proportion of the living colonies of these species were 70-2200 cm diameter, while most of the dead corals were 20-60 cm diameter (Fig. 3B). Most other species were 10-40 cm in diameter, with a slight (non-significant) increase in larger colonies between 1998 and 2005, possibly due to growth of these species over the 8 year study (Fig. 4; Table 1).
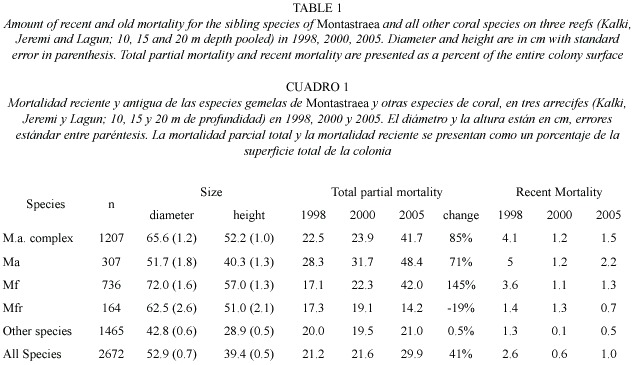
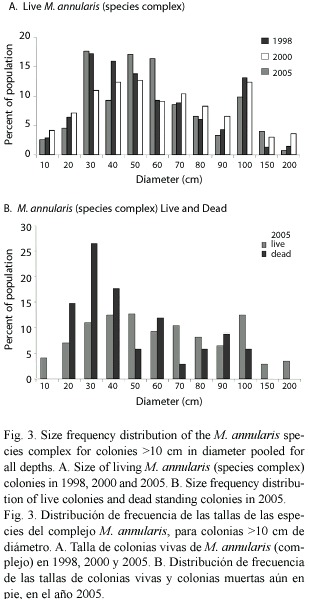
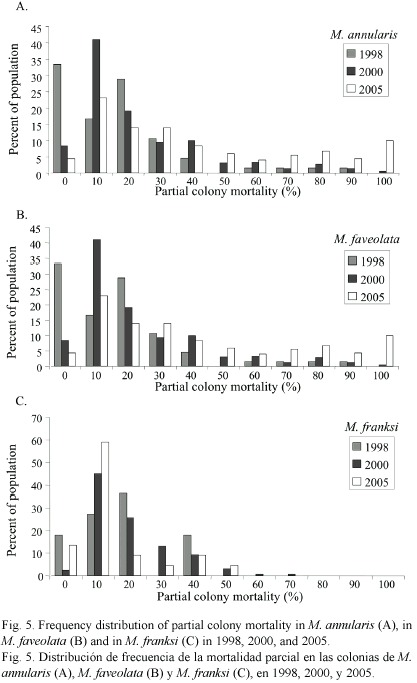
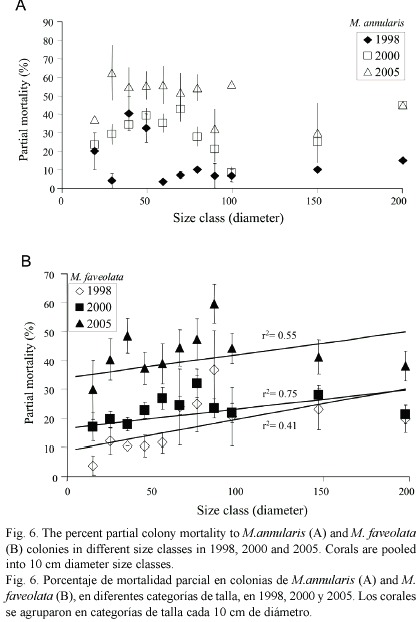
Condition of corals between 1997-2005: The percent partial tissue loss on scleractinian corals (pooled species) increased from 21% to 30% between 1998 and 2005. M. annularis (complex) sustained the largest losses (85% increase), while no significant increase in partial tissue loss was observed for all other species (Table 1). Montastraea annularis and M. faveolata sustained significantly larger losses than M. franksi, with living colonies exhibiting 42-48% partial tissue mortality, and numerous (6% of all examined) dead colonies distributed throughout the reef in 2005 (Fig. 5). There was no significant correlation between size and amount of partial mortality for M. annularis (r2= 0.05; p=0.4), while the percent mortality increased with size for colonies of M. faveolata between 10 and 100 cm diameter in 1998 (p=0.001), 2000 (p=0.009) and 2005 (p=0.01) (Fig.6).
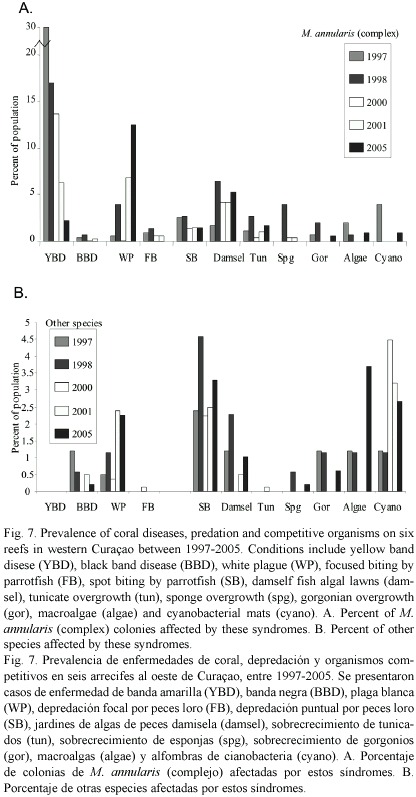
Causes of mortality: The most significant sources of colony mortality observed during this study were yellow band disease (YBD) and white plague (WP), with losses compounded by the 1998 bleaching event, damage associated with wave surge from Hurricane Lenny in 1999 and Hurricane Ivan in 2004, fish bites from Sparisoma viride, Stegastes planifrons (three spot) damselfish algal lawns, overgrowth by tunicates (Trididemnum), sponges (Cliona delitrix) and gorgonians (Erythropodium caribaeorum) and an increase in mats of cyanobateria, Schizothrix spp. Montastraea annularis an M. faveolata were affected more often and more severely by these factors than all other corals (Fig.7)
YBD has affected from 3-49% of all M. annularis (complex) colonies within transects conducted on western reefs between 1997 and 2005. The highest prevalence of YBD occurred in 1997 and 1998, and the numbers of new infections declined since 2000. YBD affected larger corals more frequently than the small corals (mean diameter of corals with YBD= 72 cm; mean diameter of unaffected corals = 61 cm), with only the largest corals showing signs of YBD in 2005. Colonies with YBD showed signs of the disease in one or more locations when first tagged in 1997, but tissue mortality was restricted to a relatively small portion of the colony surface (<10%). Over time, YBD lesions developed in multiple locations and these progressively radiated outward across the coral, eventually coalescing with other bands. Spreading rates of YBD ranged from about 5-12 cm per month between 1998-2000 (all tagged corals pooled), and 3-5 cm/yr between 2000-2005 (surviving corals with active YBD in 2005). Over 21% of the colonies tagged with YBD between 1997-1999 were still affected in 2005. Of the remainder, 43.7% died, 2.3% were affected by other diseases and 31.6% experienced partial mortality and signs of YBD have disappeared. Both colonies of M. annularis and M. faveolata in remission and colonies that still showed active signs of YBD had lost over 90% of their tissue, with remnant patches remaining primarily at the base of colonies (M. faveolata) and on adjacent lobes (M. annularis); colonies of M. franksi with YBD were missing on average 30% of their tissue.
White plague occurred at a relatively low frequency (2%) or was absent from transects examined in 1997, 1998 and January 2000. A higher frequency of WP and occurred from about 5 m to over 30 m depth in June 2001, and a second outbreak was identified in June 2005. The disease moved across colonies at rates of 2-5 cm per day, with numerous colonies along transects exhibiting 50-70% recent tissue loss when first examined. The highest frequency of WP was observed among M. annularis and M. faveolata, although WP affected 14 other species; infections were aggregated in small patches (1-3 m2 area), but were distributed throughout all depths.
Patterns of recovery: The only signs of regrowth observed among tagged YBD corals has been in M. franksi. These corals lost considerably less tissue (mean <30%) from YBD than the other two species, and some colonies exhibited resheeting over lesions. In the other species, unaffected portions of the colonies (e.g., adjacent lobes in M. annularis) continued to grow upward, but only the smallest lesions healed. Exposed skeletal surfaces were colonized by macroalgae (predominantly Lobophora, Dictyota and Halimeda) and cyanobacteria (Schizothrix), encrusting invertebrates (Trididemnum, Erythropodium, Millepora, Cliona delitrix and other unidentified sponges) and corals. Dead skeletal surfaces of tagged colonies (73.7%) were colonized by at least one coral recruit, with some colonies having up to 11 recruits (mean 2.5 recruits/coral). Recruits included ten scleractinain coral species and one hydrozoan coral, with a dominance by A. agaricites (43.6%) and P. astreoides (34%). A higher number of recruits and higher diversity of recruiting species was observed on Kalki Reef (mean= 3.9 recuits per coral), and the lowest on Jeremi Reef (mean= 1/coral).No M. annularis (complex) recruits were observed within transects or on exposed skeletal surfaces over the duration of this study.
Discussion
Coral reefs examined off Curaçao have undergone significant changes over the last three decades (Bak and Nieuwland 1995, Bruckner and Bruckner 2003) and have continued to decline over the duration of this study. Acropora palmata and A. cervicornis were prevalent in the 1970s, and declined in the early 1980s as a result of a regional disease epizootic (Van Duyl 1985); these species were present at low abundances at the eastern end of the island (e.g., Ostpunt), but were not observed within transects at the western end over the eight year study (Bruckner and Bruckner 2003). At the beginning of this study, leeward reefs from 5-20 m depth were dominated by intermediate to large-sized massive scleractinian taxa, with M. faveolata, M. annularis , and M. franksi collectively making up nearly half or more than half of all corals. While the population abundance of M. franksi has not changed, the abundance of M. annularis and M. faveolata has been reduced, with large numbers of standing dead or nearly dead (>90% tissue loss) colonies observed in 2005 and living colonies exhibiting increasing amounts of partial mortality. Living coral cover ranged from 17-49% in 1997, and has not declined substantially within any of the sites examined, with the exception of a few shallow sites (5 m depth) that lost >95% of the live corals as a result of wave surge associated with hurricane Lenny in 1999 (Bruckner and Bruckner 2003). In other sites, A. agaricites, P. astreoides, M. meandrites, Madracis decactis and other corals have colonized exposed reef substrates and denuded M. annularis colony surfaces.
Most colonies over 25 cm (69%) were missing at least 10% of their tissue, with a mean partial mortality of 22% when first examined in 1998. The amount of partial mortality differed among species, and was highest in the slow growing, large, massive broadcast spawners (M. annularis complex, S. siderea, S. intersepta, C. natans) that dominated these reefs (Bruckner and Bruckner 2003). Over time, the amount of partial mortality has increased substantially among M. annularis and M. faveolata, with colonies missing on average over 40% of their tissue in 2005, while M. franski and all other species have mean amounts of partial mortality that is similar or less than that observed in 1998. Lower amounts of partial mortality in these species may be partially related to a higher capacity for regeneration of lesions (especially lesions in M. franksi caused by fish bites), as well as an increase in the number of small colonies of other species (e.g., agaricids and poritids) with little or no mortality.
High densities of large colonies with relatively low amounts of partial tissue mortality, as observed on Curaçaos reefs in the mid-1990s, were indicative of environmentally stable old growth conditions. However, this may no longer be the case for these reefs, as recent disease epizootics have inundated these reefs and certain dominant species. The three outbreaks of disease reported in this study occurred shortly after other disturbances, including a bleaching event and two storm events, which may have stressed the host species. The prevalence of these emerging diseases and other syndromes may be at least partially related to the overall composition and diversity of coral communities and the relative abundance of M. annularis (complex). High densities of M. annularis and M. faveolata may increase the likelihood of rapid spread of infectious diseases because of the close proximity of colonies.
Increasing rates and extent of mortality in M. annularis and M. faveolata was primarily associated with recent outbreaks of YBD and WP, with localized damage from two recent hurricanes. Curaçaos reefs were first affected by an outbreak of YBD in 1995 (Hoetjes pers. comm.). In 1997, the highest prevalence was observed at the eastern end of the island where it affected 30.6% of M. annularis (complex) colonies at Piscadera; 49%, at Jan Thiel and 37.5%, at Oostpunt, while reefs off western Curaçao had a mean prevalence of 24% (Bruckner and Bruckner 2003). There were very few new YBD infections observed in 2000, and only the largest colonies with extensive areas of previously exposed skeleton showed signs of YBD in 2005, suggesting that these infections were very old. At the peak of the YBD epizootic, larger colonies of M. annularis (complex) were infected with YBD more frequently than small colonies (Bruckner and Bruckner 2003). YBD caused much slower rates of mortality than that observed in other common Caribbean diseases, but individual colonies have shown signs of the disease for up to eight years, and infections often emerge in multiple locations, exacerbating overall rates of mortality. Colonies with active signs of YBD in 1998 had lost a mean of 44% of their tissue area in January 2000, or roughly twice that of uninfected colonies of the same species (Bruckner and Bruckner 2003). About 44% of the tagged corals identified with YBD in 1997 and 1998 were dead in 2005, 21% were still affected by YBD, and nearly one-third of the colonies were in remission, although most had lost >90% of their tissue.
An outbreak of a condition resembling white plague type II (WP) was first observed in 2001, with a second outbreak observed in 2005. This disease is reported to kill coral tissue at rates of 1-2 cm per day or more (Richardson and Aronson 2003). During the Curaçao outbreaks, infected corals were observed throughout all reefs and depths. Many colonies with WP exhibited recent losses of 50-70% of their tissue, with the greatest amount of tissue loss in colonies that appeared to be relatively unblemished prior to becoming infected. The disease did not appear to discriminate among species (Weil 2004); it was documented on 14 species of corals in Curaçao, but it most frequently affected M. annularis and M. faveolata.
Colonies of Montastraea spp. are known to reaccrete tissue and skeletons over lesions (Meesters et al. 1996, Bruckner and Bruckner 2002) but resheeting may be prevented if other competitors settle on exposed skeletal surfaces or other stressors compromise the coral. On the reefs examined in Curaçao recovery through regrowth is being hindered by several compounding factors, including bleaching-related mortality, predation by parrotfish, damselfish algal lawns, overgrowth by invertebrates, and the presence of dense mats of macroalgae and cyanobacteria. Colonies affected by YBD have also subsequently exhibited signs of WP and BBD, and a few colonies have also shown signs of up to four diseases (YBD, WP, DSD and BBD) simultaneously, similar to observations reported by Weil (2004) in other parts of the Caribbean. Furthermore, the exposed skeletal surfaces of diseased corals have provided substrates supporting recruitment of many important reef-building genera, including Eusmilia, Porites, Agaricia, Diploria and Colphophyllia, but not Montastraea. Exposed substrates are also being colonized by "pest species" such as the tunicate Trididemnum, boring and encrusting sponges and the encrusting gorgonian Erythropodium.
Given the importance of the sibling species of Montastraea as the current principal frame-builders at shallow and intermediate depths, recent impacts from disease are unprecedented and may have serious ramifications for the persistence of M. annularis (complex). These corals are "bet-hedgers"; they live for centuries, requiring many years before first reproduction, and have low larval recruitment rates, even though a large number of gametes are produced on an annual basis (Szmant-Froelich 1985). The prevalence of large, hundreds of year old colonies of M. annularis (complex) and few colonies less than 30 cm in diameter suggests that recruitment events of significance have not occurred among these species in several decades. Because of the slow rates of tissue growth, colonies with large injuries from fast-spreading diseases like WP have a high likelihood of impaired skeletal growth or diminished reproductive output. Continued tissue loss and fission may further reduce the reproductive potential of these colonies as the colony diverts more energy to maintenance, and the proportion of small, non-breeding colonies in the population may increase. If additional corals die, several decades or more may be required for their replacement because of their slow rate of growth, delayed reproduction, and infrequent, episodic recruitment (Szmant 1991).
The reefs of Curaçao have undergone major changes over the last eight years, largely due to a decline in the condition of M. annularis and M. faveolata colonies. Much of these changes can be attributed to an outbreak of YBD in the late 1990s and outbreaks of WP in 2001 and 2005. Reefs may recover from disease impacts if conditions improve. Apparently healthy colonies of M. annularis (complex) with little partial mortality are still abundant, a relatively small proportion of the population of these species has died, and most of the surviving corals that were affected by disease have remnant patches of living tissue that could resheet over lesions. Additional efforts need to be directed towards understanding the causes of these diseases, factors contributing to their occurrence and spread, and long term effects on coral community structure, in order to begin developing feasible options to mitigate disease impacts (Woodley et al. 2003). It is imperative that we also continue to monitor coral diseases and other biotic agents on reefs of Curaçao, and pay close attention to the M. annularis (complex) due to their importance to the reef structure in this region.
Acknowledgments
This study was supported by funding obtained through the University of Puerto Rico, NOAA Fisheries Office of Habitat Conservation, and a Presidential Early Career Award (PECASE) in 2003. Logistical support was provided by CARMABI and All West Divers (Curaçao) and the Black Durgon Inn and the Bonaire Marine Park (Bonaire). We are grateful for field assistance provided by Al Catafulmo, Errol Coombs, Michelle Sharer, and Jim Rosborough. The opinions and views expressed in this document are those of the authors and do not necessarily reflect those of the National Marine Fisheries Service, National Oceanic and Atmospheric Administration, or the U.S. Government.
Resumen
Los arrecifes someros a sotavento del oeste de Curaçao están dominados por extensas poblaciones de Montastraea annularis (complejo). Estas especies son mayores en tamaño (promedio= 66 cm de diámetro) que las otras especies, con algunas colonias pequeñas (<30 cm) y una notable ausencia de reclutas. En 1998, las colonias de Montastraea annularis (complejo), representaban más del 45% de todas las especies de >10 cm observadas en transectos y la mayoría exhibió bajos niveles de mortalidad parcial (prom= 22.5%). En el 2005, fueron menos abundantes (38% de las colonias). La mortalidad parcial en colonias vivas de M. annularis y M. faveolata se incrementó en un 85% (promedio= 42% de mortalidad parcial) y se observaron numerosas colonias de M. annularis y M. faveolata muertas; las colonias de M. franski generalmente se encontraron en excelentes condiciones (14% de mortalidad parcial). Se documentó una alta prevalencia de enfermedades de coral (3-30%) en M. annularis y M. faveolata, mientras que las otras especies se vieron afectadas con menor frecuencia. La enfermedad de banda amarilla (BA) emergió poco después del blanqueamiento de 1995 y se dispersó rápidamente en todas las profundidades, alcanzando su mayor prevalencia entre 1997-1999. La BA causó mortalidades lentas (= 1cm/mes), con aparición de múltiples lesiones focales en colonias individuales; estas lesiones fueron creciendo progresivamente al tiempo que mataban las colonias. Para el año 2005, el 44% de los corales que se etiquetaron habían muerto; los restantes exhibían infecciones activas de BA (21%), o estaban en remisión (31.6%) pero habían perdido un promedio de >90% de su tejido. Aunque la incidencia de BA disminuyó a partir del 2000, la prevalencia de la plaga blanca (PB) fue inusualmente alta (4-12%) en el 2001 y 2005, provocando mortalidades recientes de hasta un 70% en las colonias afectadas. Las superficies muertas de Montastraea spp. han sido colonizadas por otros corales, incluyendo porítidos, agarícidos y otros fávidos, mientras que continúa la ausencia de reclutas de M. annularis (complejo). Si las enfermedades y otros estresores bióticos persisten en estos arrecifes, las poblaciones de M. annularis y M. faveolata podrían decaer de forma similar a como lo hicieron los acropóridos del Caribe durante la década de 1980.
Palabras clave: monitoreo de corales, enfermedad de corales, enfermedad de banda amarilla, plaga blanca, coral estrella, Montastraea annularis (complejo), Curaçao, Caribe.
References
Adey, W. H. & R. B. Burke. 1976. Holocene bioherms (algal ridges and bank-barrier reefs) of the eastern Caribbean. Geol. Soc. Am. Bull. 87: 5-109. [ Links ]
Aronson R. B. & W. F Precht. 2001. Evolutionary paleo-ecology of Caribbean coral reefs, p. 171-233. In W. D. Allmon & D. L. Bottjer (eds.). Evolutionary Paleoecology: The Ecological Context of Macroevolutionary Change. Columbia Univ. Press, New York, New York, USA. [ Links ]
Bak, R. P. M. & M. S. Engel. 1979. Distribution, abundance and survival of juvenile hermatypic corals (Scleractinia) and the importance of life history strategies in the parent coral community. Mar. Biol. 54: 41-52. [ Links ]
Bak, R. P. M. & G. Nieuwland. 1995. Long-term change in coral communities along depth gradients over Leeward reefs in the Netherlands Antilles. Bull. Mar. Sci. 56: 609-619. [ Links ]
Bruckner, A. W. & R. J. Bruckner. 2002. Coral predation by Sparisoma viride and lack of relationship with coral disease. Proc. 9th Int. Coral Reef Symp., Bali 2: 1245-1251. [ Links ]
Bruckner, A. W. & R. J. Bruckner. 2003. Condition of coral reefs off less developed coastlines of Curaçao (stony corals and algae). Atoll Res. Bull. 496: 370-393. [ Links ]
Bruckner, A. W. 2003. Proceedings of the Caribbean Acropora workshop: potential application of the Endangered Species Act as a conservation strategy. NOAA Tech. Memo. NMFS-OPR-24 January 2003. Silver Spring, Maryland, USA. 199 p. [ Links ]
Bythell, J. C., E. Gladfelter & M. Bythell. 1993. Chronic and catastrophic natural mortality of three common Caribbean corals. Coral Reefs. 12: 143-152. [ Links ]
Cervino J., T. J. Goreau, I. Nagelkerken, G. W. Smith & R. Hayes. 2001. Yellow band and dark spot syndromes in Caribbean corals: distribution rate of spread cytology and effects on abundance and division rate of zooxanthellae. Hydrobiol. 460: 53-63. [ Links ]
Dustan, P. 1975. Growth and form in the reef-building coral Montastrea annularis. Mar. Biol. 33: 101-107. [ Links ]
Geister, J. 1977. The influence of wave exposure on the ecological zonation of Caribbean reefs. Proc. 3rd Int. Coral Reef Symp., Miami 1: 23-29. [ Links ]
Gil-Agudelo D. L., G. W. Smith, J. Garzón-Ferreira, E. Weil & D. Peterson. 2004. Dark spots disease and yellow band disease, two poorly known coral diseases with high incidence in Caribbean reefs, p. 337-350. In E. Rosenberg & Y. Loya (eds.). Coral Health and Disease. Springer, Berlin. [ Links ]
Glynn, P. W 1973. Ecology of a Caribbean coral reef. The Porites reef-flat biotope: Part I. Meteorology and hydrography. Mar. Biol. 20: 297-318. [ Links ]
Goreau, T. F 1959. The ecology of Jamaican coral reefs. I. Species composition and zonation. Ecology 40: 67-990.
Goreau, T. F. & J. W. Wells. 1967. The shallow-water Scleractinia of Jamaica: Revised list of species and their vertical distribution range. Bull. Mar. Sci. 17: 442-453. [ Links ]
Goreau, T. F & N. I. Goreau. 1973. The ecology of Jamaican coral reefs. II Geomorphology, zonation, and sedimentary phases. Bull. Mar. Sci. 23: 399-464. [ Links ]
Green, E. & A. W. Bruckner. 2000. The significance of coral disease epizootiology for coral reef conservation. Biol. Conserv. 96: 347-361. [ Links ]
Highsmith, R. C., R. L. Lueptow & S. C. Schonberg. 1983. Growth and bioerosion of three massive corals on the Belize barrier reef. Mar. Ecol. Prog. Ser. 13: 261-271. [ Links ]
Hudson, J. H. 1981. Growth rates in Montastrea annularis: A record of environmental change in Key Largo National Marine Sanctuary, Florida. Bull. Mar. Sci. 31: 444-459. [ Links ]
Jackson, J. B. C. 1992. Pleistocene perspectives on coral reef community structure. Amer. Zool. 32: 719-731. [ Links ]
Jordán-Dahlgren E. & R. E. Rodríguez-Martínez. 2004. Coral diseases in Gulf of Mexico Reefs, p. 105-118. In E. Rosenberg & Y. Loya (eds.). Coral Health and Disease. Springer, Berlin. [ Links ]
Knowlton, N., E. Weil, L. A. Weigt, & H. M. Guzmán. 1992. Sibling species in Montastraea annularis, coral bleaching, and the coral climate record. Science 255: 330-333. [ Links ]
Kojis, B. L. & N. J. Quinn. 1994. Biological limits to Caribbean reef recovery: A comparison with Western South Pacific reefs. Proc. Colloq. Global Aspects of Coral Reefs, RSMAS, Univ. Miami. Pp. 353-359. [ Links ]
Kramer, P. R. & J. C. Lang. 2003. The Atlantic and Gulf Rapid Reef Assessment (AGRRA) Protocols: former version 2.2. Appendix One. In J.C. Lang (ed.). Status of Coral reefs in the Western Atlantic: Results of initial surveys, Atlantic and Gulf Rapid Reef Assessment (AGRRA) Program. Atoll Res. Bull. 496: 611-624. [ Links ]
Liddell, W. D. & S. L. Ohlhorst. 1986. Changes in benthic community composition following the mass mortality of Diadema in Jamaica. J. Exp. Mar. Biol. Ecol. 95: 271-278. [ Links ]
Meesters E. H., I. Wesseling & R. P. M. Bak. 1996. Partial mortality in three species of reef-building corals and the relation with colony morphology. Bull. Mar. Sci . 58: 838-852. [ Links ]
Nugues, M. M., I. Nagelkerken, K. Vermonden & O. C. C. Moraes. 2005. Spatial and temporal variations in the prevalence of coral diseases in the Caribbean island of Curaçao. 32nd Scient. Meet. AMLC. Abstract. [ Links ]
Richardson, L. L. 1998. Coral diseases: what is really known. Trends Ecol. Evol. 13: 438-443. [ Links ]
Richardson, L. L. & R. A. Aronson. 2002. Infectious diseases of reef corals. Proc. 9th Int. Coral Reef Symp., Bali 2: 1225-1230. [ Links ]
Santavy, D. L., E. C. Peters, C. Quirolo, J. W. Porter & C. N. Bianchi. 1999. Yellow-blotch disease outbreak on reefs of the San Blas Islands, Panama. Coral Reefs (Reef Sites) 18: 97. [ Links ]
Stoddart, D. R. 1963. Catastrophic storm effects on the British Honduras reefs and cays. Nature 196: 512- 515. [ Links ]
Sutherland, K. P., J. W. Porter & C. Torres. 2004. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar. Ecol. Prog. Ser. 266: 273-302. [ Links ]
Szmant-Froelich, A.M. 1985. The effect of colony size on the reproductive ability of the Caribbean coral Montastraea annularis (Ellis and Solander). Proc. 5th Int. Coral Reef Symp., Tahiti 4: 295-300. [ Links ]
Szmant, A. M. 1991. Sexual reproduction by the Caribbean reef corals Montastraea annularis and M. cavernosa. Mar. Ecol. Prog. Ser. 74: 13-25. [ Links ]
Van Duyl, F. C. 1985. Atlas of living reefs of Curaçao and Bonaire. Ph.D. isser., Free Univ. Amsterdam/Found. Scient. Res. Surinam Netherlands Antilles, Utrecht, Netherland. 37 p.
Weber, J. N. & E. W. White. 1977. Caribbean reef corals Montastraea annularis and Montastraea cavernosa: Long term growth data as determined by skeletal X-radiography, p. 171-179. In S. H. Frost, M. P. Weiss & J. B. Sanders (eds.). Reefs and Related Carbonates- Ecology and Sedimentology. Amer. Assoc. Petrol. Geol., Studies in Geology No 4. [ Links ]
Weil, E. 2004. Coral reef diseases in the wider Caribbean, p. 35-668. In E. Rosenberg & Y. Loya (eds.). Coral Health and Disease. Springer, Berlin.
Weil, E. & N. Knowlton. 1994. A multi-character analysis of the Caribbean coral Montastraea annularis (Ellis and Solander, 1786) and its two sibling species, M. faveolata (Ellis and Solander, 1786) and M. franksi (Gregory, 1895). Bull. Mar. Sci. 55: 151-175. [ Links ]
Woodley, C. M., A. W. Bruckner, S. B. Galloway, S. M. McLaughlin, C. A. Downs, J. E. Auth, E. B. Shotts & K. L. Lidie (eds.). 2003. Coral Disease and Health: A National Research Plan. National Oceanic and Atmospheric Administration, Silver Spring, Maryland, USA. 72 p. [ Links ]
Internet reference
Hoetjes, P. 2003. Re: coral disease outbreak-remote Western Caribbean atoll. Coral-list posting on Friday May 30, 2003 (coral-list@coral.aoml.noaa.gov, downloaded August 2006). [ Links ]












 uBio
uBio