Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.54 n.1 San José Mar. 2006
Characterization of a Bacillus thuringiensis strain collection isolated from diverse Costa Rican natural ecosystems
Glen Arrieta1 & Ana M.Espinoza1,2
1 Centro de Investigación en Biología Celular y Molecular (CIBCM),Universidad de Costa Rica, Sabanilla de Montes de Oca, San José, Costa Rica; garrieta@costarricense.cr
2 Escuela de Agronomía, Facultad de Ciencias Agroalimentarias, Universidad de Costa Rica, San José, Costa Rica; amespino@racsa.co.cr
Received 18-IV-2005. Corrected 21-VII-2005. Accepted 20-IX-2005.
Abstract: Costa Rican natural ecosystems are among the most diverse in the world. For this reason, we isolated strains of the entomopathogenic bacteria Bacillus thuringiensis (Bt ) to determine their diversity, distribution and abundance. A total of 146 Bt strains were obtained from environmental samples collected from diverse natural ecosystems and life zones of Costa Rica. We recovered Bt strains from 71%, 63%, 61% and 54% of soil samples, fresh leaves, other substrates and leaf litter respectively. Bt was isolated in 65% of the samples collected in the humid tropical forest in national parks (Braulio Carrillo, Gandoca Manzanillo, Sierpe, Hitoy Cerere, and Cahuita), and in 59% of the samples collected in the dry tropical forest (Parque Nacional Marino las Baulas, Palo Verde and Santa Rosa). In the very humid tropical forest (Tortuguero) Bt was isolated in 75% of the samples and in the very humid tropical forest transition perhumid (Carara) it was found in 69% of the samples. The strains exhibit a diverse number, size and morphology of parasporal inclusion bodies: irregular (47%), oval (20%), bipyramidal (3%), bipyramidal and cubic (1%), bipyramidal, oval and irregular (5%) and bipyramidal, oval and cubic crystals (2%). Strains isolated from Braulio Carrillo, Tortuguero and Cahuita, presented predominantly irregular crystals. On the other hand, more than 60% of the isolates from Térraba-Sierpe and Hitoy-Cerere had medium oval crystals. Strains from Gandoca-Manzanillo, Palo Verde and Carara presented mainly combinations of oval and irregular crystals. Nevertheless, the greatest diversity in crystal morphology was observed in those from Santa Rosa, Llanos del Río Medio Queso and Parque Marino las Baulas. Protein analyses of the crystal-spore preparations showed 
Key words: Bacillus thuringiensis, crystals, cry, vip genes, 
Costa Rica is one of the 20 richest countries in terms of biodiversity, harboring 4% of the total biodiversity of the world (Obando 2002). The geographic location of the country in the neotropics, its geology, two closely separated coasts, a complex mountain range system and diverse microclimates and ecosystems are some of the determining factors for the biological diversity of the country. The neotropics provide a rich source for the discovery of new species and strains of microorganisms. Therefore prospecting for gene diversity is envisage as a promising investigative area of the 21st century, giving Costa Rica a unique opportunity to lead the process in the region. The genetic resources obtained from microorganisms play an important role in the production of new enzymes, antibiotics and bioinsecticides by the biotechnological industry (Bull et al. 1992, Samsonov et al.1997).
It has been estimated that 67 000 species of plagues affect agriculture in the world, and approximately 9 000 species are insect pests (Ross and Lembi 1985). As a result, sustainable control of insects in agriculture is crucial since, for 2001, it was estimated that chemical worldwide control of insects cost 7 500 million dollars (James 2002). In addition, the use of synthetic insecticides is not recommended because of the undesirable effects on human health, ecological problems caused by their slow degradation and the lack of specificity in their insecticidal action. This situation has stimulated the search of new alternatives of insect control based on the entomopathogenic bacteria Bacillus thuringiensis (Bt ).
Bt is a Gram positive bacteria of the Bacillaceae family that has been used as a bioinsecticide for the biological control of plagues of economic importance in agriculture over the last decades (Aronson et al. 1986). This bacteria synthesizes crystalline insecticidal proteins or 
Most Bt strains produce 
The identification of Bt cry and cyt genes by PCR has proven to be a very useful method for strain characterization, offering several advantages in terms of rapidity and reproducibility (Ben-Dov et al . 1997, Porcar and Juárez- Pérez 2003). A single Bt strain can harbor up to eight different cry genes (Martínez 2002). In general, the type of cry and cyt genes present in a strain correlates to some extent with its insecticidal activity (Porcar and Juárez-Pérez 2003). Thus, the identification of the gene profile in a Bt collection can be a useful tool to predict its potential insecticidal activity.
Since Costa Rican insects diversity is estimated to be 360 000 species (Obando 2002), and because a co-evolution of Bt strains and their susceptible insect hosts has been proposed (Apoyolo et al. 1995), prospecting for Bt strains in diverse natural ecosystems could result in the identification of Cry proteins with new specificities. The objective of this research was to isolate and characterize a collection of Bt strains from natural ecosystems, representing the diverse life zones of Costa Rica. The results obtained offered information about the prevalence of Bt in the country and the distribution, abundance and diversity of Bt strains. Those strains could be used in the near future in the formulation of insecticides for the biological control of insects of economic importance for the country. In addition, their cry genes could be used for the genetic transformation of plants.
Materials and methods
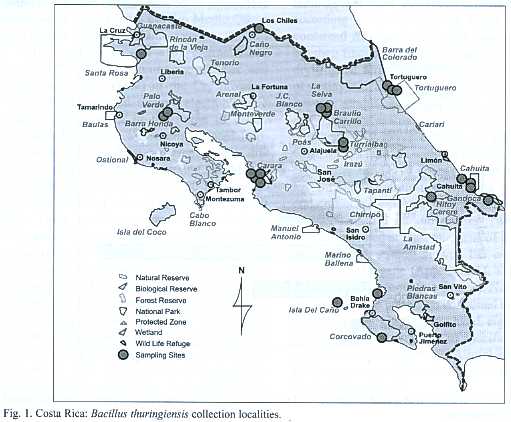
Sample collection: Two hundred and sixty five environmental samples (soil, leaf litter, fresh leaves and other substrates)were collected from protected areas that include the diverse ecosystems and life zones of Costa Rica (Fig.1).One hundred samples were collected from the humid tropical forest (Braulio Carrillo, Gandoca Manzanillo, Sierpe, Hitoy Cerere, and Cahuita), 80 from the dry tropical forest (National Park Marino las Baulas, Palo Verde, Santa Rosa), 24 of the very humid tropical forest (Tortuguero) and, finally, 61 samples from the humid tropical forest transition perhumid (Carara).The samples were dried at 50 ºC for 24 hours and stored at room temperature.
Isolation of Bt : Bacteria were isolated using the protocol described by Travers et al.(1987) using T3 as selective enrichment medium. Sporulated cultures showing the typical Bt morphology were preserved both on filter paper at room temperature and in 50% glycerol at-70 ºC.
Light microscopy: Cultures of approximately five days were analyzed by light microscopy (Nikon E-200 Eclipse) by staining with Coomassie blue (0,25%(w/v) in 60% ethanol (v/v) and 7% of acetic acid (v/v), with the purpose of determining crystal morphology.
Polyacrylamide gel electrophoresis: Crystal-spore preparations were analyzed for the presence of the 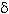
DNA extractions: The protocol for DNA extraction described by Chen and Kuo (1993) was used and DNA concentrations were estimated by fluorometry at 280 nm (Quantech fluorometer, model FM1 109535).
PCR analyses: The general primer for the cry1, cry3-7, cry5, cry8, cry11, cry12, cry14 , and cyt genes and specific primer for cry1Aa, cry1Ab,cry1Ac,cry1Ad,cry1B,cry1C,cry1D, cry1E,cry1F (Bravo et al., 1998), also vip1 , vip2,vip3 (A. Bravo, personal communication) and the general primer for cry2 gene (Ben-Dov et al.1997) were used. For the PCR the following conditions were used: 25 mM MgCl2,10x buffer, 10mM dNTPs, 20 µM each primer, 2.5 U Taq polymerase and 5 to 20 ng of DNA.The PCR program was: one denaturing cycle of two min. at 95 °C, 30 cycles of one min. at 95 °C, one min. at 48-54 °C, one min. at 72 °C, and a final extension cycle of 5 min. at 72 °C. PCR products were analyzed by gel electrophoresis in 1%(w/ v)agarose gels. The reference strains HD-137, HD-1, Btt, HD-916, were obtained from the Bacillus Genetic Center Stock; Department of Biochemistry (Ohio State University).
Results
A total of 146 Bt strains were isolated from environmental samples from diverse natural ecosystems collected from 9 of the 12 life zones of Costa Rica.Bt strains were obtained from 60% of the samples. It was possible to recover Bt strains with an efficiency of 71%, 63%, 61% and 54% from soil samples, fresh leaves, other substrates and leaf litter respectively. Bt was isolated in 65%of the samples collected in the humid tropical forest of the national parks (Braulio Carrillo, Gandoca Manzanillo, Sierpe, Hitoy Cerere, and Cahuita), in 59% of the samples collected in the dry tropical forest (Parque Nacional Marino las Baulas, Palo Verde and Santa Rosa). In the very humid tropical forest (Tortuguero) Bt was isolated in 75% of the samples and in the very humid tropical forest transition perhumid (Carara) was found in 69% of the samples (Fig.1). Statistical analysis (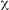
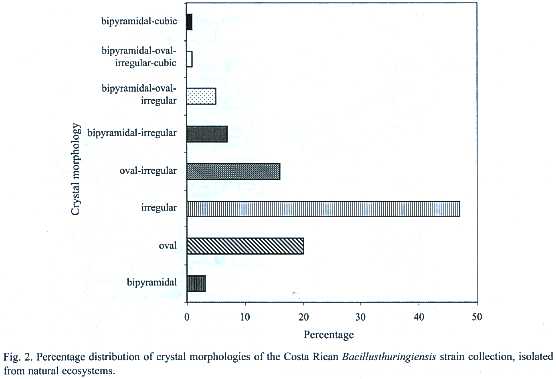
The microscopic observation of the crystals showed high diversity in the morphology, number as well as in the size of the parasporal inclusions. Small, medium and large oval crystals were frequently found. Also, some strains presented small and large bipyramidal as well as irregular crystals (Fig. 2). This diversity was reflected also in the presence of different crystal inclusions,since some strains produce crystals with several morphologies,whereas others show only one type of crystal. Most strains presented more than one crystal morphology, for example up to six different crystals in the same strain. Figure 2 shows the different crystal morphologies, the irregular being the most common (47%), followed by the oval (20%). Twenty-four strains (16%) showed a combination of both.The combination of bipyramidal and cubical crystals was rare.
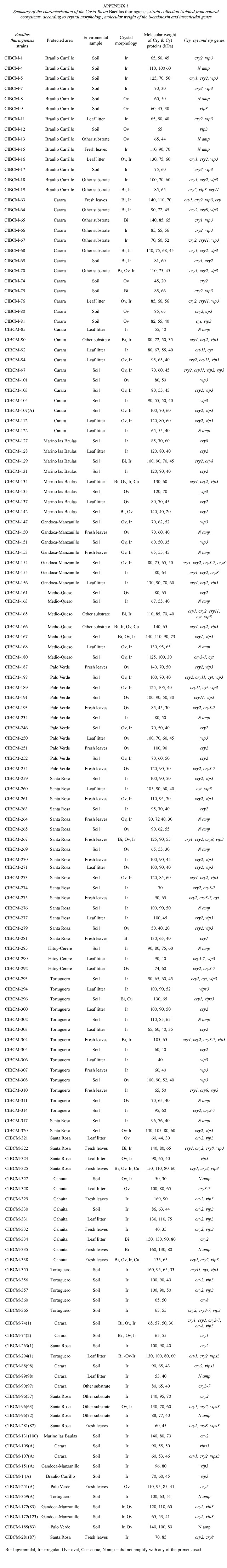
No correlation was found when crystal morphology and isolation sites were compared. However, strains isolated from Braulio Carrillo, Tortuguero and Cahuita, presented abundant irregular crystals. One of the few strains that presented large cubic, oval and bipyramidal crystals was isolated from Tortuguero. On the other hand, more than 60% of the isolated strains of Térraba-Sierpe, and Hitoy-Cerere presented medium oval crystals. Several strains isolated from Gandoca-Manzanillo, Palo Verde and Carara presented mainly combinations of oval and irregular crystals.The greatest diversity in morphologies was observed in those from Santa Rosa, Llanos del Río Medio Queso and Parque Marino las Baulas. The collection was also characterized by SDS-PAGE to determine the number and molecular weight (MW)of the Cry proteins. The analyses showed diverse electrophoretic patterns,with MWs in the range of 20 to 160 kDa (Appendix 1).Strains isolated from Parque Marino las Baulas presented very different 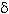
The characterization of the collection by PCR showed that cry2 and vip3 genes were found in 87 and 85 strains respectively. Thirty- one strains amplified with the general primer cry1 ,14 with cry3-7, 12 with cry8, 11 harbored the cry11 gene and finally 13 amplified with the cyt general primer. Amplification with the general primers for the cry5, cry12, cry14 and vip1 genes was not detected. Also it is important to mention that 24 strains did not amplify with any of the primers used. Figure 3 shows the distribution of the genes of the collection according to forest type.All types of forest contained Bt strains with same type of genes, but their frequencies differed. Nevertheless, the dry tropical forest showed the greatest number of strains with cry2, cry3-7, cry8 and cyt genes. Several strains presented great diversity of cry genes,for example CIBCM-165 amplified with cry1,cry2,cry11,cyt and vip3 genes.Also CIBCM-154 amplify with the primers for cry1, cry2,cry3-7 and cry8 genes (Appendix 1).
Strains that amplified with the cry1 general primer were further analyzed with specific primers for different subfamilies. Twenty-two strains presented at least one gene of the cry1A subfamily,11 strains showed the cry1D and four the cry1B gene (Appendix 1).The cry1E gene was scarce (only two strains). None of the Bt strains of this collection contained the cry1C or cry1F genes. On the other hand,eight strains did not amplified with any of specific primers tested for cry1 subfamilies. A total of 13 different genetic profiles were found for the cry1 family (Appendix 1).
Discussion
A collection of 146 strains of Bacillus thuringiensis was obtained from environmental samples derived from diverse natural ecosystems of Costa Rica.This research corroborated the broad distribution of Bt in different microhabitats. Isolation efficiency was of 60%, a very similar figure to that reported by other authors (Martin and Travers 1989). It is important to indicate that the dry tropical forest seems to present the greatest diversity, not only in crystal morphologies but also in the insecticidal protein genes.
This collection showed great diversity in morphology,size and number of parasporal inclusions. It was not possible to establish a correlation between crystal morphology and the origin of the strain.Although it was possible to isolate strains from different areas with similar crystal morphologies, it is interesting to emphasize that some strains from specific sites presented rare morphologies, for example the CIBCM-142 from the Parque Marino las Baulas showed long bipyramidal crystals and CIBCM- 296 from Tortuguero had large cubic crystals.
Diversity in crystal protein patterns was also revealed by electrophoresis.Our results differed from those obtained in strains isolated from agricultural ecosystems (Víquez 2000). It was reported previously that the characterization by SDS-PAGE of these strains showed homogeneous 
The Bt crystals of some strains are made up of a single protein, for example Bt var kurstaki HD-73 that contained only the Cry1Ac protein. Some of the characterized strains of this collection, showed a single protein in the SDS-PAGE, suggesting that their crystals are comprised of a single protein, or by two or more proteins with the same molecular weight. Nevertheless, one type of crystal could be constituted by different proteins as in the strain Bt var morrisoni, where proteins Cry4, Cry1A and Cyt form several inclusions covered by a common membrane (Ibarra et al. 1986). In other strains, like the HD-1, several proteins (Cry1Aa,Cry1Ab,and Cry1Ac) form a single crystal (Hofte et al. 1998). Abundant oval crystals were observed in the strain CIBCM-251, but when analyzed by electrophoresis, three 
It was also noticed that strains with bipyramidal crystals frequently presented two proteins, one of high MW of 120 or 140 kDa and another of 65 or 60 kDa. These proteins could be the protoxin and the active toxin respectively. As Bt regulates the synthesis of proteases that can process protoxin to toxins (Rukmini et al. 2000), the proteolysis could imply reduction of activity, since the 
Some of the 
The most common cry genes found in nature are those within the cry1 family (Porcar and Juárez-Pérez 2003). In our collection strains containing cry1 genes were not so abundant (20%). The cry2 gene was the most frequently found, like in other collections (Ibarra, personal communication).It has been reported that these proteins have MWs of 70 to 75 kDa. Table 1 shows that several strains of the collection expressed a protein of 70 kDa that could be the Cry2 protein,a protein toxic for insects of the Lepidoptera and Diptera orders.The high frequency of these proteins in several Bt strains could have permitted them to extend the host range.
The second most frequent gene of this collection was vip3, (58 %). Estruch et al .(1996) found this gene in only 15% of the strains of their collection. Arrieta et al. (2004) also reported a widespread distribution of this gene in strains isolated from Costa Rican agricultural ecosystems. In contrast, the vip1 gene was not detected in this collection and only one strain presented the vip2 gene. Different results were obtained in a Costa Rican collection isolated from coffee plantations where the frequency of the vip2 gene was higher (Arrieta et al. 2004).The occurrence of cry1 gene varies greatly among different Bt collections. For example cry1A genes were frequently present in more than 50% of the strains, whereas other genes of the subfamily, such as cry1E and cry1F, were less frequent. However there are some exceptions, such as the high frequency of cry1E in a Chinese collection (Porcar and Juárez-Pérez 2003).
Several reports showed a high frequency of certain combinations of cry1 genes, for example the linkage of the cry1C and cry1D genes (Bravo et al. 1998, Ferrandis et al.1999, Hongyu et al. 2000).This cry1C and cry1D linkage may be explained by their location on the same replicon (Sanchis et al. 1988). However, eleven Bt strains of the Costa Rican collection contained only the cry1D. Ferrandis et al.(1999) suggested that the absence of the cry1C gene might be explained by a deletion or negative selection of the cry1C gene from an ancestral cry1C-cry1D linkage.
In summary, the diversity of Bt toxins indicate that the collection analyzed has great potential for the control of different species of insect pests of economic importance. Strains with a diversity of genes for the control of lepidopterans, dipterans and coleopterans were found. It is interesting to mention that this Bt collection obtained from natural ecosystems presented greater diversity of cry genes in comparison to other collections isolated from Costa Rican agricultural ecosystems (Arrieta et al. 2004, Mora and Espinoza 2005).The characterization of this collection will offer very useful information for the selection of Bt strains with particular Cry protein profiles to be evaluated for their toxicity against specific insect pests of important food crops.
Acknowledgments
We thank César Rodríguez for his collaboration during the initial phases of this project and to acknowledge the Fundación para la Cooperación Costa Rica-Estados Unidos de América (CR.USA) and the Programa de Cooperación del Gobierno de México for the financial support of the project. We also want to express our gratitude to CYTED for supporting the Bt-network from which our laboratory is part and to the Sistema Nacional de Areas de Conservación for the access to the environmental samples. Finally, we thank Alejandra Bravo (Institute of Biotechnology, UNAM-Mexico) for providing us with the reference strains HD-137, HD-1, Btt, HD-916.
Resumen
Como los ecosistemas naturales de Costa Rica figuran entre los más diversos del mundo, se propuso aislar la bacteria entomopatógena Bacillus thuringiensis (Bt ) con el fin de conformar una colección de cepas y caracterizarlas molecularmente. Se obtuvieron 146 cepas a partir de muestras ambientales de diversas áreas protegidas, que incluían 9 de las 12 zonas de vida de Costa Rica. Se recobraron cepas del 71%, 63%, 61%y 54% de las muestras de suelo, hojas frescas, otros sustratos y hojarasca respectivamente. Se aisló Bt del 65% de las muestras del bosque tropical húmedo, un 59% de las muestras del bosque tropical seco. Del bosque tropical muy húmedo se aisló Bt del 75% de las muestras y finalmente del bosque tropical muy húmedo transición perhúmedo se encontró en el 69% de las muestras. Las cepas se caracterizaron según la morfología de los cuerpos paraesporales de inclusión, el peso molecular de las 
Palabras clave: Bacillus thuringiensis, cristales, genes cry, vip, 
References
Apoyolo, C.I., L. Drif, J.M. Vassal, H. Debarjac, J. P. Bossy, F. Leclant & R. Frutos. 1995. Isolation of multiple subspecies of Bacillus thuringiensis from a population of the European sunflower moth, Homoeosoma nebulella. Appl. Environ. Microbiol. 61: 4343-4347. [ Links ]
Aronson, A. I., W. Beckman & P. Dunn. 1986. Bacillus thuringiensis and related insect pathogens. Microbiol. Rev.50:1-24. [ Links ]
Arrieta, G., A. Hernández & A. M. Espinoza. 2004. Diversity of Bacillus thuringiensis strains isolated from coffee plantations infested with the coffee berry borer Hypothenemus hampei Ferrari. Rev. Biol. Trop. 52:757-764. [ Links ]
Ben-Dov, E., A. Zaritsky, E. Dahan, Z. Barak, R. Sinai, R. Manasherob, A. Khamraev, E. Troitskaya, A. Dubitsky, N. Berezina & Y. Margalith. 1997. Extended screening by PCR for seven cry -group genes from field-collected strains of Bacillus thuringiensis. Appl. Environ. Microbiol. 63: 4883-4890. [ Links ]
Betz F., B. G. Hammond & R. L. Fuchs. 2000. Safety and advantages of Bacillus thuringiensis protected plants to control insects pest. Reg. Tox. Pharm. 32: 156-173. [ Links ]
Bravo, A., S. Sarabia, L. López, H. Ontiveros, C. Abarca, A. Ortiz, M. Ortiz, L. Lina, J. Villalobos, G. Peña, V. G. Noez, M. Soberon & R. Quintero. 1998. Characterization of cry genes in a Mexican Bacillus thuringiensis strain collection. Appl. Environ. Microbiol. 64: 4965-4972. [ Links ]
Bull, A. T., M. Goodfellow & J. H. Slater. 1992. Biodiversity as a source of innovation in biotechnology. Ann. Rev. Microbiol. 46: 219-252. [ Links ]
Chen, W. & T. Kuo. 1993. A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucl. Acids Res. 21: 2260. [ Links ]
Crickmore, N., D. R. Zeigler, J. Feitelson, E. J. Schnepf Van Rie, D. Lereclus, J. Baum & D. H. Dean. 1998. Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiolol. Mol. Rev. 62: 807-813. [ Links ]
Estruch J. J., G. W. Warren, G. J. Mullins, G. C. Nye, J. A. Craig & M. G. Koziel. 1996. Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectum of activities against lepidopteran insects. Proc. Natl. Acad. Sci. USA 93:5389-5394. [ Links ]
Ferrandis, M. D., V. M. Juárez-Pérez, R. Frutos, Y. Bel & J. Ferré. 1999. Distribution of cry1, cryII, and cryV genes within Bacillus thuringiensis isolates from Spain. System Appl. Microbiol. 22: 179-185. [ Links ]
Hofte, H. & H. R. Whiteley. 1989. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol. Rev. 53: 242-255. [ Links ]
Hongyu, Z., Y. Ziniu & D. Wangxi. 2000. Composition and ecological distribution of Cry proteins and their genotypes of Bacillus thuringiensis isolates from ware-houses in China. J. Invertebr. Pathol. 76: 191-197. [ Links ]
Ibarra, J. E. & B. A. Federici. 1986. Parasporal bodies of Bacillus thuringiensis ssp.morrisoni (PG-14)and Bacillus thuringiensis ssp. israeliensis are similar in protein composition and toxicity. FEMS Microbiol. Lett. 34: 78-84. [ Links ]
James, C. 2002. Global review of commercialized transgenic crops: 2001 feature: Bt cotton. International Service For The Acquisition of Agri-Biotech Application. 26. [ Links ]
Martin, P. A. & R. S. Travers. 1989. Worldwide abundance and distribution of Bacillus thurigniensis isolates. Appl. Environ. Microbiol. 55: 2437-2442. [ Links ]
Martínez, C. 2002. Biological and genetic characterization of Bacillus thuringiensis strains toxic against Helicoverpa armigera. 573 p. UPNA.Pamplona. [ Links ]
Mora, R. & A. M. Espinoza. 2005. Isolation and characterization of Bacillus thuringiensis strains isolated from insects of the order Homoptera. Rev. Biol. Trop. (Submitted). [ Links ]
Obando. 2002. Biodiversity in Costa Rica: state of knowledge and management. INBio-SINAC. San José, Costa Rica. [ Links ]
Porcar, M. & V. Juárez-Pérez. 2003. PCR-based identification of Bacillus thuringiensis pesticidal crystal genes. FEMS Microbiol. Rev. 26: 419-432. [ Links ]
Ross, M. A. & C. A. Lembi. 1985. Applied Weed Science. 340 pages. McMillanNew York, New York, USA. [ Links ]
Rukmini, V., Y. R. Reddy & G. Venkateswerlu. 2000. Bacillus thuringiensis crystal 
Samsonov, P., R. I. Padrón, C. Pardo, J. Cabrera & G. A. De la Riva. 1997. Bacillus thuringiensis from biodiversity to biotechnology. J. Industri. Microbiol. Biotech. 19:202-219. [ Links ]
Sanchis, V., D. Lereclus, G. Menou, J. Chaufaux & M. M. Lecadet. 1988. Multiplicity of 
Travers, R. S., P. A. W. Martin & C. F. Reichelderfer. 1987. Selective process for efficient isolation of soil Bacillus spp .Appl. Environ. Microbiol. 53: 1263-1266. [ Links ]
Víquez, A. 2000. Búsqueda y caracterización de proteínas biopesticidas novedosas a partir de cepas de Bacillus thuringiensis aisladas de ecosistemas costarricenses. Tesis Facultad de Microbiología, Universidad de Costa Rica. [ Links ]