Revista de Biología Tropical
versión On-line ISSN 0034-7744versión impresa ISSN 0034-7744
Rev. biol. trop vol.54 no.1 San José mar. 2006
Common polymorphisms and cardiovascular factors in patients with myocardial infarction of Costa Rica
Lizbeth Salazar-Sánchez1, Lilliana Chaves2, Mayra Cartin3, Gudrun Schuster4, Karin Wulff4, Winnie Schröder4 & Falko H. Herrmann4
1 Centro de Investigacion en Hematología y Trastornos Afines,(CIHATA)Universidad de Costa Rica,Hospital San Juan de Dios 10634-1000,San José,Costa Rica.Tel:(506)223-1385 Fax:(506)222-158;lsalazar@cariari.ucr.ac.cr
2 Servicio de Cardiología.Hospital San Juan de Dios,San José,Costa Rica.
3 Escuela de Salud Pública,Universidad de Costa Rica.Tel:(506)207-4421,Fax (506)253-6436; mcartinb@cariari.ucr.ac.cr
4 Institut of Human Genetics,Ernst Moritz Arndt University,Fleischmannstr.42/44,D-17487 Greifswald,Germany. Tel:+49-3834-86 5371,Fax:+49-3834-86 5393; Gudrun_Schuster@gmx.de; wschroed@uni-greifswald.de; herrmafh@mail.uni-greifswald.de
Received 16-III-2005. Corrected 11-VIII-2005. Accepted 14-X-2005.
Abstract: Eight common polymorphisms of known myocardial infarction (MI)risk factors (factor V Leiden (FVL), factor V HR2 (FVHR2), factor II 20210G>A (FII), factor VII IVS7 (FVII IVS7), factor VII Arg353Gln (FVII), factor XIII Val34Leu (FXIII), Methylenetetrahydrofolate reductase C677T (MTHFR), Angiotensin Converting Enzyme (ACE))and environmental risk factors were analyzed in a MI patients of Costa Rica.This case-control study included 186 MI subjects,95 of them <45 years and 201 age and sex matched controls.With the use of PCR method the polymorphisms were detected and through interviews additional information was collected.Hypercholesterolemia and smoking were associated with a significant risk in younger patients.High fibrinogen level was an important risk factor and interaction with smoking was detected.Mainly,the genotype 34LeuLeu of FXIII showed significant protective effect,(OR 0.32,95%CI 0.13-0.80)while the other polymor- phisms showed no significant difference between the cases and the controls.Carriers of FVII (OR 2.75,95%CI 1.07-7.02)and FXIII (OR 4.20,95%CI 2.03-8.67)polymorphisms showed interaction with fibrinogen in the sta- tistical analysis.It was concluded that there was an important interaction between the common risk factors and the polymorphisms (FVII;FXIII)in the development of MI.This is one of the first reports in a Latin-American population dealing with these molecular markers and MI.Rev.Biol.Trop.54(1):1-11.Epub 2006 Mar 31.
Key words: Arterial thrombosis, myocardial infarction, polymorphisms, fibrinogen, Latin-American.
Myocardial infarction (MI)develops as result of thrombosis in coronary arteries. Numerous studies have examined the relation of common risk factors involvement in atherosclerosis as obesity, hypertension, diabetes mellitus,smoking and abnormalities in the haemostatic system in the pathogenesis of MI (Meade et al .1986,Doggen et al.1998).
Mutations of the coagulation factors: factor V Leiden (FVL),factor V gene haplotype R2 (FVHR2)and factor II G20210A ( FII 20210 G>A)are known as risk factors for thrombosis in Caucasian populations (Doggen et al.1998, Lunghi et al.1996,Ardissino et al.1999). Hyperhomocystinemia has been documented to be an independent risk factor for thromboembolic and cardiovascular disease (CVD) (Kang et al .1991),and it is often related to a thermolabile variant of the enzyme methylene- tetrahydrofolate reductase (MTHFR)(Kang et al.1991,Frosst et al.1995).Studies associated the presence (insertion,I)or absence (deletion, D)of a 287bp Alu repeat element in intron 16 of the Angiotensin Converting Enzyme gene (ACE)with the level of the circulating enzyme or cardiovascular pathophysiology (Cambien et al .1992).Some genetic variants of FVII and FXIII where reported as protective effects for thrombosis (Iacovello et al.1998, Kohler et al.1998).
Few studies have examined the effect of interaction of environmental factors and molecular markers in the risk of MI particularly among younger populations (Herrmann et al. 2001, Martini et al.2005),such as the general Costa Rican population (Morera and Barrantes 2004).The aim of this study was to identify the prevalence and impact of the common risk factors and eight polymorphism of genes affecting thrombosis in other populations (FVL,FHR2, FII 20210G>A,FVII IVS7,FVII Arg353Gln, FXIIIVal34Leu, MTHFR,ACE) in one Costa Rican case -control study.
Materials and methods
Patients and controls: The MI patients belonged to the participating centers of Security Social of Costa Rica (San Juan de Dios and Heredia Hospital).The diagnosis of MI was based on ischemic chest symptom, typical electrocardiograph (ECG) changes, and elevation of serum creatine kinase and its MB isoenzyme to more than twice the upper level of normal. Coronary angiography was performed in 125 patients.The severity of coronary atherosclerosis was determined by the number of coronary arteries with steno sis of more than 50 percent of the luminal diameter.
Controls: Samples were collected from 201 unrelated and apparently healthy subjects (hospital and university staff,students and blood donors)comparable with patients for age,sex and ethnicity.None of these controls had suffered episodes of CVD.
A complete clinical summary, with emphasis on personal and family history for MI, cardiovascular risk factors was obtained from all subjects by trained staff. A subject was considered to have arterial hypertension, diabetes or hypercholesterolemia when he or she was receiving specific medications to treat these conditions and/or when there was an established diagnosis of each disease. Information concerning with smoking habits, family history of CVD,use of oral contraceptives and body mass index (BMI) was also obtained for all subjects. Women with BMI >26 kg/m2 and men with BMI >28 kg/m2 were considered obese. Only subjects with history of regular cigarette consumption were included as smokers. Ethics local committees of participating institutions approved this study.The informed consent was obtained from all the subjects (cases and controls).
Determination of the molecular analysis: DNA was isolated from peripheral blood according to standard protocols (Miller et al. 1988). For some analyses blood samples soaked onto filter paper cards from the probands were used for polymerase chain reaction (PCR) as described previously (Schröder et al. 1996). Amplification was carried out on 50 µL volume samples in a Perkin Elmer-Cetus thermal cycler.Genotyping of FVL,FVHR2,FII 20210G>A,MTHFR,FVII Arg353Gln were performed by PCR amplification of each of the target alleles from genomic DNA followed by restriction digestion with each of corresponding enzyme Mnl I (Bertina et al.1994), Rsa I (Lunghi et al.1996),Hin dIII (Frosst et al. 1995),Hin fI (Poort et al.1996),Msp I (Green et al.1991).FVII IVS7 was analyzed by PCR amplification as previously described (O Hara and Grant 1988).The 163G>T polymorphism of the FXIII gen was analyzed by PCR amplification and heteroduplex analysis (Kohler et al.1998)ACE polymorphism was performed according to described by Rigat et al .(1992). To prevent mistyping of the DD genotype, a second PCR with insertion specific primers was performed as described previously (Odawara et al .1997)
Fibrinogen analysis: The fibrinogen was determined by Clauss assay (Clauss et al. 1957),with Fibri-Prest (Diagnostica Stago)on a DiaMed-CD4 coagulometer.Normal ranges were:200 to 400 mg/dL.
Statistical analysis: Statistical analysis was performed by the Statistical Package Epi. Info.6 (CDC, Atlanta USA) and Stata Statistical Software: Release 6.0. College Station,TX. To compare the basic characteristics, odds ratio (ORs) with 95% confidence intervals (CIs)was calculated and 
Results
The demographic characteristics and prevalence of MI risk factors in cases and control subjects are shown in Table 1. The mean age of the males and females cases was 46.2 and 46.0 years, respectively (p =0.910). Of note, 51.1% of the cases were 
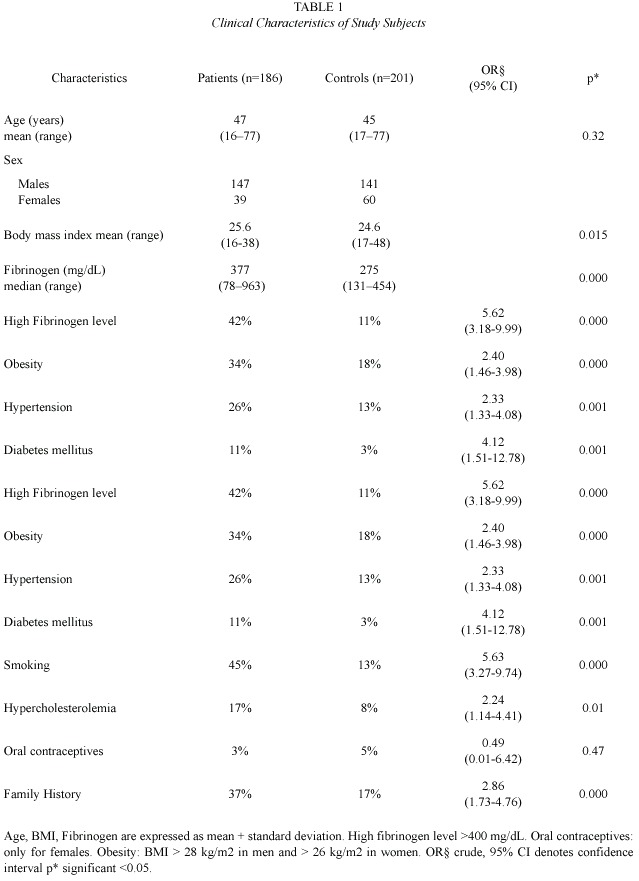
Coronary artheriography was performed in 125 cases (67%),38 (30%)of whom had normal coronary arteries, 30 (24%) had 1 vessel disease, 26 (21%)had 2 vessel disease, and 31 (25%) had 3 or more vessel disease. There was no association between common risk factors, polymorphisms and the degree of coronary artery disease. The only statistically significant association was with age (p =0.000).In the older group (>45 years 87%)arterial plaques were more frequently than in the younger group (<45 years, 59%).
Association in the risk of MI and the level of fibrinogen was investigated by stratification analyses among cases and controls. The risk of MI increased with increasing levels of fibrinogen. None of the control individuals had fibrinogen level >500 mg/dl. It was not possible to obtain the OR for this fibrinogen level, but this category had the highest value for 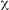
Prevalence of the genetic polymorphisms: The genotype distribution was in the Hardy-Weinberg equilibrium in the case and control group. The FXIII 34LeuLeu genotype detected in 12.20% of controls which was significantly higher than 4.60% observed in cases. No significant differences in the allele frequencies of the others analyzed polymorphisms in the patient and control group was observed, Table 2. Furthermore, uncommon monomers (4,5 and 8) of FVII IVS7 were found in the controls, without statistical significance.
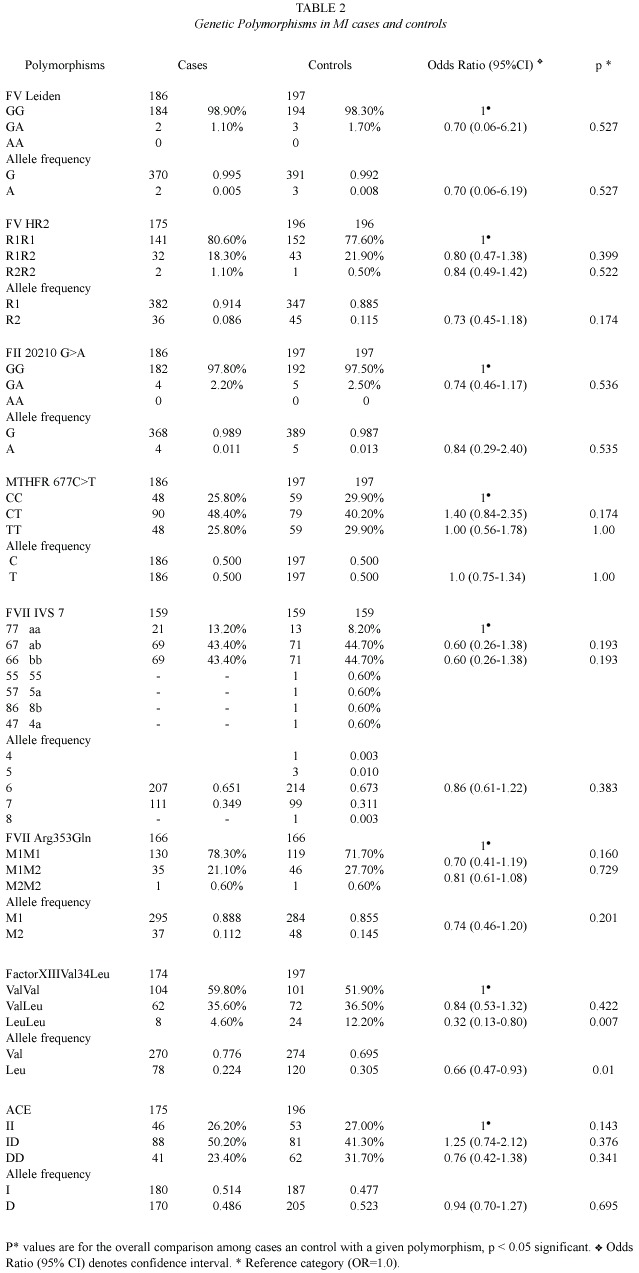
Adjustments were made for sex age and select risk factors using unconditional logistic regression. The results are given in the Table 3. Briefly, the risk factors significantly associated with MI were: fibrinogen levels, smoking, diabetes mellitus, family history, obesity and hypertension.
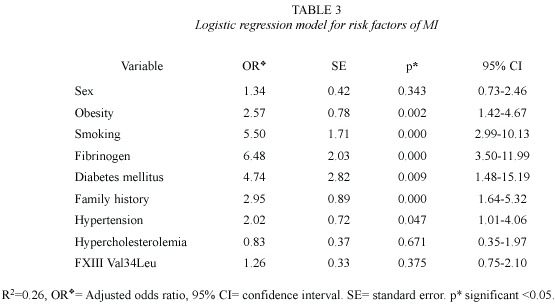
Interaction analysis: Interaction between smoking and age (p =0.01) was observed. Smoking was confirmed as a risk factor of MI,it was stronger in the younger group (OR 10.45,95% CI 5.10-21.37, p <0.000)than in the older group (OR 2.85,95% CI 1.38-5.88, p =0.004). Hypercholesterolemia was associated with a significant risk only in the younger group (OR 6.21,95% CI 1.93-26.05, p =0.000). Elevated fibrinogen level had a significant risk in both groups,but the association as risk factor was stronger in the older group (OR 12.18, 95% CI 4.69-37.72, p <0.000). The Leu allele of FXIII Val34Leu polymorphism was found with protective effect in the younger group (OR 0.55,95% CI 0.33-0.95,p =0.01).
Combined analysis risk factors and genetic polymorphisms: Logistic regression and stratified analyses detected the importance of the co-occurrences of some of the genotypes and the major risk factors. The risk of MI associated with smoking and fibrinogen was modified by the carriership of FVII polymorphisms: in the carriers subjects of FVII IVS and FVII Arg353 decreased the risk 4 fold (Table 4). Also, carriers subjects of FVII and FXIII decreased the risk of MI. It was detected:3.5-fold in carriers of FVII IVS7 (aa,ab);2-fold for FVII Gln353 and 1.5- fold for FXIII Leu34 (Table 4).
There was no interaction between carriers and non-carriers of FVII IVS7 (a)and FXIII Val34Leu polymorphisms and the remaining risk factors. Concerning of FVHR2, ACE, MTHFR, polymorphisms were not found statistical significant between carriers or no carriers in relation to the presence or absence of the common risk factors.Interaction of FVL and the FII G20210A were excluded,no homozygotes for either FII 20210A or FVL were found in both groups.
Discussion
Over the past years remarkable progress has been made concerning the role of common atherogenic factors in the pathogenesis of MI. The results of our study, in which we examined the role of eight molecular markers and common risk factors as well as the interaction genetic and non genetic factors in 186 MI patients, 51% of them were younger than 45 years old, confirm that genetic variability rather to have direct role, maybe influences a predisposition give by non genetic factors to MI.
The major coronary risk factors for our younger patients, mainly men, were smoking- hypercholesterolemia and smoking-fibrinogen. These factors are consistently and independently related to cardiovascular risk (Meade et al. 1986, Hunter et al. 2001, Fedder et al.2002).The evidence is based on numerous prospective epidemiological studies and clinical observations in the general population (Hunter et al. 2001, Fedder et al. 2002, Koenig 2003).However, the relations age-smoking and age-cholesterol observed give a special point to improve treatment and prevention guidelines in our younger group of the Costa Rican population.
However, the reasons, why fibrinogen is elevated and the combination smoking-high fibrinogen are only incompletely understood. Some studies discussed the participation of cytokines, such as interleukin 6, in the stimulation of the transcriptional activity involved in the increase of fibrinogen synthesis as well as mediators of the inflammatory response in smokers (Hunter et al. 2001, Koenig 2003). Smoking "per se "is a prothrombotic stimulus, because it is able to increase fibrinogen,also smokers exhibit a mild,but sustained, acute-phase response,characterized by increased plasma concentrations of positive acute-phase protein (Hunter et al. 2001). Whether or not fibrinogen is causally involved in atherothrombogenesis still remains to be determined.
More than half of our patients had non significant coronary artery stenosis or single vessel disease, that could be explained by the age of the group studied and also the possible role of genetics and the common risk factors found. Family history was a risk factor for MI, as a higher prevalence of familial myocardial infarction was found among cases (37%) compared with controls (17%) without any risk factors, leading to an OR 2.86 (95%CI 1.73,4.76). This increased risk was recently described in a study as possible relation with socio-economic circumstances, lifestyle or an undiscovered genetic influence (Martini et al.2005).
In our data, the FXIIIVal 34 Leu polymorphism was the only genetic factor associated with MI. The difference in carrier frequency for the Leu allele was significant between control group and patients. The protective effect of FXIII 34 Leu allele in the younger cases was not previously reported and suggests that FXIII 34 Leu allele may impart a protective effect for MI in the age matched control group.
None of the remaining genetic markers evaluated were associated alone with the risk of MI. There are several explanations for these results: First-could be attributing to the population-specific factors, the low frequency of some of this polymorphism in the CR population (i.e.,documented differences in the geographic distribution of carriers of this polymorphism (Herrmann et al. 2001) and other polymorphisms (Azofeifa et al. 2004, Chaves-Villalobos et al .2004, Morera et al. 2001,2003). Second-previous studies presented conflicting results in the association of some of the analyzed markers as risk factor for MI (i. e., FVL, MTHFR, ACE and FII G20210A (Doggen et al.1998, Ardissino et al.1999, Kang et al. 1991, Frosst et al. 1995, Cambien et al.1992). In addition, we failed to observe an independently protective effect, of the alleles of Gln353 (M2)allele and the 7(a)repeats of the FVII polymorphisms as was reported in older patients which positive family history of CVD (Cambien et al.1992).
It was possible observed gene-environmental factors interactions. A decreased risk in smokers who carry FVII polymorphisms was detected,this result is consistent with the protective effect of these polymorphisms in smokers reported by Iacovello (Iacovello et al. 1999). In addition,we observed interactions not previously reported between carriers of 7 (a)repeats allele of FVII IVS7,Gln 353 allele and Leu 34 of FXIII Val34Leu polymorphisms with high fibrinogen levels as protective effect. Carriers of 7(a)repeats of FVII IVS7 with high fibrinogen levels present a significant decreased in the risk of MI.Decrease of the risk was also detected in carriers of FVII 353Gln and in carriers of 34Leu of FXIII polymorphism who had high fibrinogen level.
This is the first report of these polymorphisms and conventional risk factors in MI in a Latin-American (LA) country. Further studies with more patients as well as other LA populations are need to confirm and clarify the possible role of these genetic-environmental interactions in the risk for MI in this population.
Acknowledgment
This work was made possible by the support from the Faculty of Medicine, Ernst-Moritz-Arndt- University, Greifswald Germany; Deutscher Akademischer Austauschdienst (DAAD)and by Vicerrectoría de Investigación, University of Costa Rica (project No.807-A3-903).
Resumen
Se estudiaron ocho polimorfismos comunes asociados como factores de riesgo para el infarto al miocardio (IM):factor V Leiden (FVL),factor VHR2 (FVHR2), factor II 20210G>A (FII),factor VII IVS7 (FVII IVS7), factor VIIArg353Gln (FVII),factor XIIIVal34Leu (FXIII), metilentetrahidrofolato reductase C677T (MTHFR), enzima convertidora de la angiotensina (ACE) y factores ambientales de riesgo,en pacientes costarricenses.Este es un estudio de casos y controles,donde participan 186 pacientes,95 de ellos con edades <45 años y 201 sujetos controles.Se utilizó la técnica de reacción en cadena de la polimerasa (PCR)y por medio de entrevistas personales se recolectó información epidemiológica adicional.Se encontró que la hipercolesterolemia y el fumado estan asociados como factores de riesgo en los pacientes jóvenes.Niveles elevados del fibrinógeno fueron detectados como un factor de riesgo importante y se observo interacción entre fumado y estos valores aumentados de fibrinógeno. El genotipo 34LeuLeu del FXIII presentó un efecto protector significante mientras que los otros polimorfimos estudiados no mostraron diferencia estadísticamente significativa entre los casos y controles. Los polimorfismos del FVII y FXIII demostraron interación con el fibrinógeno,según el análisis estadístico aplicado. Se evidencia, la interación entre factores de riesgo común y ciertos polimorfismos (FVII;FXIII)en la patogénesis del IM.Este es uno de los primeros informes sobre estos marcadores moleculares y su asociación con IM en una población latinoamericana.
Palabras clave:Trombosis arterial,infarto al miocardio infarction, polimorfismos, fibrinógeno, Latinoamérica.
References
Ardissino,D.,P.M.Mannucci,P.A.Merlini,F.Duca,R. Fetiveau,L.Tagliabue,M.Tubaro,M.Galvani,F. Ottani,M.Ferrario,J.Corral &M.Margaglione. 1999.Prothrombotic genetic risk factors in young survivors of myocardial infarction.Blood 94:46-51. [ Links ]
Azofeifa,J.,M.Hahn,E.Ruiz,L.Hummerich,A.I. Morales,G.Jiménez &R.Barrantes.2004.The STR polymorphism (AAAAT)n within the intron 1 of the tumor protein 53 (TP53)locus in 17 populations of different ethnic groups of Africa,America,Asia and Europe.Rev.Biol.Trop.52:645-657. [ Links ]
Bertina,R.M.,B.C.Koeleman,T.Koster,R.F.Rosendaal, R.J.Dirven,H.de Ronde,P.A.van der Velden &P.H. Reitsma. 1994.Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 369:64-67. [ Links ]
Cambien,F.,O.Poirier,L.Lecerf,A.Evans,J.P.Cambou, D.Arvelier,G.Luc,J.M.Bard,L.Bara,S.Ricard, L. Tiret, P. Amouyel, F. Alhenc-Gelas & F. Soubrier. 1992.Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature 359:641-644. [ Links ]
Chaves-Villalobos,M.,G.Jiménez-Arce &M.Sandí-Díaz. 2004.Polimorfismo del gen de la banda 3 eritrocítica en grupos étnicos de Costa Rica.Rev.Biol.Trop.52: 659-663. [ Links ]
Clauss,A.1957.Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogen. Acta Haematol.17: 237-246. [ Links ]
Doggen,C.J.,V.M.Cats,R.M.Bertina &F.R.Rosendaal. 1998.Interaction of coagulation defects and cardiovascular risk factors:increased risk of myocardial infarction associated with factor V Leiden or prothrombin 20210A.Circulation 97:1037-1041. [ Links ]
Fedder, D.O., C.E. Koror & G.J.L Italien. 2002. New national cholesterol education program III Guidelines for primary prevention Lipid-Lowering drug therapy. Projected impact on the size,sex and age distribution of the treatment-eligible population.Circulation 105: 152-156. [ Links ]
Frosst,P.,H.J.Blom,R.Milos,P.Goyette,C.A.Sheppard, R.G.Matthews,G.J.Boers,M.den Heijer,L.A.J. Kluijmans,L.P.van den Heuvel &R.Rozen.1995.A candidate genetic risk factor for vascular diseases:a common mutation in methylentetrahydrofolate reductase.Nat.Genet.10:111-113. [ Links ]
Green, F.,C.Kelleher,H.Wilkes,A.Temple,T.Meade & S.Humphries.1991. A common genetic polymorphism associated with lower coagulation factor VII level in healthy individuals. Arterioscler. Thromb. 11:540-546. [ Links ]
Herrmann,FH,L.Salazar-Sanchez,W.Schröder,R. Grimm,G.Schuster,G.Jimenez-Arce,M.Chavez & J.R. Singh. 2001. Prevalence of molecular risk factors FV Leiden,FVHR2,FII20210G>A and MTHFR 677C>T in different populations and ethnic groups of Germany, Costa Rica and India.Int.J.Hum.Genet. 1:33-39. [ Links ]
Hunter,K.A.,P.J.Garlik,I.Broom,S.E.Andersons & M.A.McNurlan.2001.Effects of smoking and abstention from smoking on fibrinogen synthesis in human.Clin.Sci.100:459-465. [ Links ]
Iacoviello,L.,A.Di Castelnuovo,A.D Orazio &M.B. Donati.1999.Cigarette smoking doubles the risk of myocardial infarction in carriers of a protective polymorphism in the blood coagulation factor VII gene. Thromb. Haemost.81:658. [ Links ]
Iacoviello,L.,A.Di Castelnuovo,P.De Knijff,A.D Orazio, C.Amore, R. Arboretti, C. Kluft & M. B. Donati. 1998. Polymorphisms in the coagulation factor VII gene and the risk of myocardial infarction. N.Engl. J.Med.338:79-85. [ Links ]
Kang S.S.,P.W.Wong,A.Susmano,J.Sora,M.Norusis &N.Ruggie.1991.Thermolabile methylenetetra- hydrofolic acid reductase:an inherited risk factor for coronary artery disease.Am.J.Hum.Genet.48: 536-545. [ Links ]
Koenig,W.2003.Fibrin(ogen)in cardiovascular disease: an update.Thromb.Haemost.89:601-609. [ Links ]
Kohler,H.P.,M.H.Stickland,N.Ossei-Gerning,A.Carter, H.Mikkola &P.J.Grant.1998.Association of a common polymorphism in the factor XIII gene with myocardial infarction.Thromb.Haemost.79:8-13. [ Links ]
Lunghi,B.,L.Iacoviello,D.Gemmati,M.G.DiIasio,E. Castoldi,M.Pinotti,G.Castaman,R.Redaelli,G. Mariani,G.Marchetti &F.Bernardi.1996 Detection of new polymorphic markers in the factor V gene: Association with factor V levels in plasma .Thromb. Haemost.75:45-48. [ Links ]
Martini, C. H., C. J. M. Doggen, C. Cavallini, F. R. Rosendaal & P. M. Mannucci. 2005. No effect of polymorphisms in prothrombotic genes on the risk of myocardial infarction in young adults without cardiovascular risk factors. Thromb. Haemost. 3: 177-179. [ Links ]
Meade, T.W., M. Brozovic, R.R.Chakrabarti,A.P.Haines, J. D. Imeson, S. Mellows, G. J. Miller, W. R. S. North, Y. Stirling &S.G.Thompson.1986.Haemostatic function and ischaemic heart disease:principal results of.the Northwick Park Heart Study.Lancet 2:533-537. [ Links ]
Miller,M.,D.D.Dykes &H.F.Polesky.1988.A simple salting out procedure for extracting DNA from human nucleated cells.Nucleic.Acids.Res.16:121. [ Links ]
Morera,B.&R.Barrantes.2004.Is the Central Valley of Costa Rica a genetic isolate?Rev.Biol.Trop.52: 629-644. [ Links ]
Morera,B.,R.Marín-Rojas &R.Barrantes.2001. Análisis de varios marcadores genéticos clásicos en la población de Costa Rica.Rev Biol Trop.49: 1237-1252. [ Links ]
Morera,B.,R.Marín-Rojas &R.Barrantes.2003.Gene Admixture in the Costa Rican Population.Ann.Hum. Genet.67:71-80. [ Links ]
O Hara,P.J.&F.J.1988.Grant The human factor VII gene is polymorphic due to variation in repeat copy num- ber in a minisatellite.Gene 66:147-158. [ Links ]
Odawara,M.,A.Matsunuma &K.Yamashita.1997. Mistyping frequency of the angiotensin-converting enzyme gene polymorphism and an improved method for its avoidance.Hum.Genet.100:163-166. [ Links ]
Poort,S.R.,F.R.Rosendaal,P.H.Reitsma &R.M.Bertina. 1996.A common genetic variation in the3 ´-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis.Blood 88:3698-3703. [ Links ]
Rigat,B.,C.Hubert,P.Corvol &F.Soubrier.1992.PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP 1)(dipeptidyl carboxypepetidase 1).Nucl.Acids. Res. 20:1433. [ Links ]
Schröder,W.,M.Koesling,K.Wulff,M.Wehnert &F.H. Herrmann.1996.Large-scale screening for factor V Leiden mutation in a North-Eastern German population.Haemostasis 26:233-236. [ Links ]












 uBio
uBio 

