Revista de Biología Tropical
versión On-line ISSN 0034-7744versión impresa ISSN 0034-7744
Rev. biol. trop vol.52 no.4 San José dic. 2004
Photochemical efficiency of adult and young leaves of the neotropical understory shrub Psychotria limonensis (Rubiaceae)in response to changes in the light environment
Gerardo Avalos1,2 &Stephen S.Mulkey3
1 The School for Field Studies,Center for Sustainable Development Studies,10 Federal St.,Salem,MA 01970 USA.
2 Escuela de Biología,Universidad de Costa Rica,2060 San José,Costa Rica.Fax:(506)207-4216; gavalos@cariari.ucr.ac.cr
3 Department of Botany,University of Florida 220 Bartram Hall,PO 118526 Gainesville,FL 32611-8526 USA.
Received 09-VIII-2002. Corrected 10-IX-2003. Accepted 28-XI-2003.
Abstract:We explored the short-term adjustment in photochemical efficiency (Fv /Fm )in adult and young leaves of the understory neotropical shrub Psychotria limonensis Krause (Rubiaceae)in response to rapid changes in the light environment.Leaves were collected from 20 individual plants growing under sun and shade conditions on Gigante Peninsula,Barro Colorado Natural Monument (Republic of Panama),during the wet season of 1996. Leaves were distributed in four sequences of light treatments (AB leaves were expanded under sun and were transferred to shade,BA leaves experienced the opposite transfer,and the controls AA and BB leaves that were expanded and maintained under sun or shade conditions).Adult and young leaves did not differ in overall photochemical efficiency.Instead,differences were found among light environments,for which leaves transferred from shade to sun showed the lowest F v /F m ratios.There was no relationship between photochemical efficiency and leaf temperature.In P.limonensis,understory plants are susceptible of photoinhibition independently of the leaf ontogenetic stage.The approach utilized in this experiment allowed the rapid exploration of this capacity, and could be applied to poorly studied understory species. Rev.Biol.Trop.52(4):839-844.Epub 2005 Jun 24.
Key words:Chlorophyll fluorescence,photoinhibition,photosynthesis,Psychotria limonensis ,tropical forest, Panama.
Light conditions of tropical forests are highly heterogeneous across successional environments (Chazdon and Fetcher 1984, Fetcher et al.1994,Chazdon et al.1996) and seasons (Wright 1996, Barone 1998). Individual leaves are exposed to transient and drastic changes in the light environment during development,and must adjust their physiology accordingly (Avalos and Mulkey 1999).Plants must adjust as well to extreme differences in light between wet and dry seasons (Kitajima et al.1997).The degree of adjustment should match the temporal and spatial heterogeneity of the habitat where the species occurs,with pioneer species expected to show a wider range of physiological plasticity relative to shade-tolerants (Bazzaz and Carlson 1982).Since basic information on light acclimation through structural and functional adjustment of whole,mature individuals is lacking for most species,metabolic responses are studied at the leaf level assuming that leaves largely regulate whole-plant responses to light changes through the control of shoot and root growth and carbon allocation (Dickson and Isebrands 1991,Field 1991).It is at the leaf level where the most critical selective pressures affecting the whole individual converge.Therefore,leaf structure and function should reflect the selective pressures acting upon the whole plant (Field 1991).
In tropical forests,understory plants are exposed to sudden changes in forest vertical structure associated with gap apertures,or continuous shading due to canopy closure. High light levels often lead to photoinhibition. Photoinhibition consists in the reduction of the maximum quantum yield of photosynthesis and the decrease in the convexity of the photosynthetic light response curve as reflected in the pattern of variable to maximum chlorophyll fluorescence emissions (Fv /Fm ratio, Demmig and Björkman 1987,Long et al.1994).The capacity to respond to such light changes at the leaf level represents a critical adaptive trait that is likely to influence the distribution and diversity of tropical species (Grubb 1977,Denslow 1980,Bazzaz and Carlson 1982,Denslow 1987,Clark and Clark 1987,Fetcher et al. 1994).The adjustment capacity depends on leaf age and structure,plant ontogeny, and the characteristics of the light environment where the leaf developed before the light change (i.e., Bauer and Thöni 1988).
Although the mechanism of photoinhibition has been largely investigated under controlled conditions (i.e.,Gouallec et al.1990, 1991,Mulkey and Pearcy 1992),there remains to be shown whether it represents a common response of understory plants to increased light levels.The current data suggest little evidence for strong and permanent damage to the photosynthetic apparatus during highlight exposure in field conditions for tropical species (Fernández and Fetcher 1991,Chazdon et al. 1996). Although moderate photoinhibition does occur,species differ in their susceptibility to become photoinhibited (Araus and Hogan 1994, Lovelock et al.1994).
In this study we compared the short-term adjustment in photochemical efficiency of adult and young leaves expanded under sun and shade conditions to different light sequences in the neotropical understory shrub Psychotria limonensis Krause (Rubiaceae).Since fluorescence emission can be affected by ambient temperature,we also explored the relationship between leaf temperature and photochemical efficiency.
The evergreen shrub P .limonensis is abundant in the understory of moist forests,from southern Mexico to northern Colombia (Croat 1978).Variation in photochemical efficiency was assessed as the time-dependent change in the ratio of variable to maximum chlorophyll fluorescence Fv /Fm ,where Fv is the difference between the maximum (Fm)and the minimal (Fo)fluorescence emissions.Variation in the Fv /Fm ratio provides a non-intrusive parameter to assess the capacity of photosystem II to use light in photosynthesis (Long et al. 1994),and constitutes a good predictor of performance under changing light conditions. Photochemical efficiency (Fv /Fm )has a high value of 0.83 in unstressed leaves (Demmig and Björkman 1987).
The study was done during the first week of August of 1996 (mid-rainy season)on Gigante Peninsula, Barro Colorado Natural Monument, in the Republic of Panama.At this site,P. limonensis is mainly associated with shaded environments,such as the understory under deep canopy cover,but it can persist under relatively high light environments, such as gap edges.The Fv /Fm ratio was measured with a portable fluorometer (Modulated Fluorometer OS-500,Opti-Sciences,Tyngsboro MA).Adult and young leaves were collected before sunrise from seven plants established under sunexposed conditions (middle of a young gap with no canopy influence)and seven plants growing under typical shade conditions (understory under deep canopy cover).Young leaves of similar size were collected from the terminal node of top branches,whereas adult,fully expanded leaves,were collected from the third node distal from the apex.Leaf petioles were immediately submerged and maintained under water using 250 ml plastic cups.Leaves were arranged in the following sequences of light treatments: AA (leaves collected from sun plants and maintained under sun conditions), AB (leaves collected from sun plants but moved to shade conditions),BA (leaves collected from shade plants but moved to sun conditions),and BB (leaves collected and maintained under shade conditions).Measurements were taken on five leaves per light sequence.Leaves were dark-acclimated for 5 min by positioning a leaf-clip with an aperture of 0.5 cm in diameter onto the leaf.The aperture was sufficiently large to allow the fiber optic probe to cover the dark-acclimated section.We used a red light pulse to completely saturate all electron acceptors. Measurements were taken every hour from 8 am to 12 m under sunny and warm conditions (27-32 ºC).Since measurements were done on the same individual leaves through time we used a Repeated Measures ANOVA to correct F values and account for reduced degrees of freedom due to the temporal autocorrelation of the response.Since Fv/Fm ratios were truncated between zero and one,values were arcsine transformed prior to analyses.Leaf temperatures were measured simultaneously relative to Fv /Fm by clipping the OS-500 leaf chamber thermocouple onto the leaf.
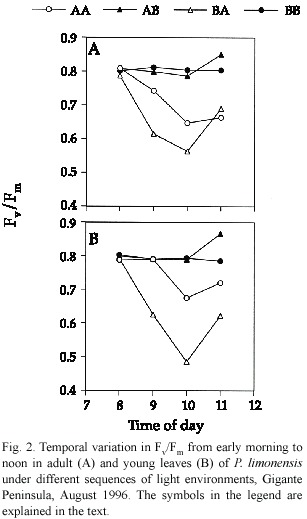
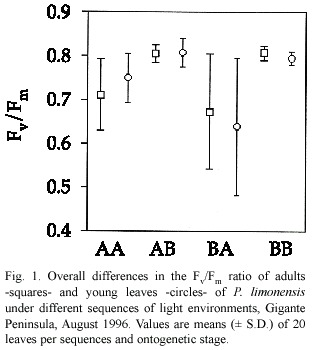
We found no differences among young and adult leaves relative to photochemical efficiency (Hotelling-Lawley Trace =0.006,P=0.72). Young leaves were expected to have lower Fv /Fm due to incomplete leaf expansion and photochemical capacity necessary to utilize most of the incident radiation.The extent of differences in overall Fv /Fm values for young and adult leaves were minimal (Fig.1).Reduction in Fv/Fm ratio was not severe across sequences, and may reflect increased levels of photoprotection,although young BA leaves showed the lowest and most variable Fv /Fm ratios,whereas leaves in the BB and AB sequences showed the highest.In this case,levels of reduction of electron carriers were low,indicating an increased opportunity to convey all the radiation during saturation pulses.The high Fv /Fm values of leaves in the AB sequence could be due to extra photochemical capacity developed within the former high light environment.Both adult and young leaves followed a similar pattern of temporal variation in Fv /Fm ,and significant differences were found only among light sequences (Hotelling-Lawley Trace =9.84,P<0.05), specially for BA leaves (Fig.2).
Variation in leaf temperature during the morning was minimal for adult (mean =28.46 ºC,sd =1.18)and young leaves (mean =29.06 ºC,sd =1.55).There were no significant differences in temperature among stages (Hotelling-Lawley =2.56,P =0.13),or sequences of light treatments (Hotelling-Lawley =0.56,P =0.58),although there was a small but significant interaction between sequence and life stage (Hotelling-Lawley =0.53,P = 0.04)reflecting the effect of time on temperature increase throughout the morning.We could expect an increase in leaf temperature to cause a decrease in Fv /Fm due to the synergistic effect of increasing temperature and photorespiration. In this case,photorespiration might act as a photoprotective mechanism,providing an alternate pathway for electron flow (Osmond 1981).
However,the low extent of differences in temperature among treatments,and the magnitude of the temperature itself,made the influence of photorespiration minimal in this case.
The results show that shade leaves of P.limonensis are prone to short-term decreases in photochemical efficiency,and are likely to suffer photoinhibition and stress due to high light, independently of their ontogenetic stage. This contrasts with data reported by Krause et al.(1995),who found higher photoinhibition in young canopy leaves.In the canopy, leaves are exposed to higher radiation levels for longer periods.Cycles of photoinhibition and recovery may reflect the role of photoinhibition as protective rather than a damaging mechanism (Krause 1988)under these circumstances. However,our data suggest a minimal influence of photoinhibition in canopy leaves, even during the dry season (Avalos and Mulkey 1999,Avalos 1999).In the understory,leaves experience lower light levels and it is likely that leaf expansion occurs mostly under shade conditions.Here,exposure to high light is more likely to lead to long term damage than in the case of canopy leaves,specially if high light exposure is combined with drought (see Lovelock et al.1994).However,P.limonensis seems to be well buffered against this condition due to its deep root system and the ability to maintain low stomatic conductance during the dry season (Wright et al.1992).
This study suggests the utility of collecting and analyzing leaves in situ by maintaining their petioles submerged in water,a technique that could be useful to perform rapid surveys of photochemical efficiency in response to rapid changes in light in poorly explored tropical species.A more detailed study,coupled with the measurement of photosynthetic capacity, is needed to fully characterize the response of P.limonensis to light changes that may take place during longer periods.
Acknowledgments
GA was supported by the International Center for Tropical Ecology at the University of Missouri-St.Louis.The authors thank the Smithsonian Tropical Research Institute for their logistic support in Panama.The School for Field Studies provided facilities for writing the manuscript.We appreciate the comments of Catherine Lovelock.Nadilia Gómez,Marla Ramos,Vanessa Sánchez,Maritzel Ramírez and Maribel López assisted during data collection.
Resumen
Se exploró el ajuste a corto plazo en la eficiencia fotosintética (Fv /Fm )en hojas jovenes y adultas del arbusto del sotobosque neotropical Psychotria limonensis Krause (Rubiaceae)en respuesta a cambios rápidos de luz ambiental. Las hojas fueron recolectadas de 20 plantas individuales bajo condiciones de sol y sombra en Peninsula Gigante, Monumento Natural Barro Colorado (Panamá),durante la estación lluviosa de 1996.Las hojas fueron distribuidas en una secuencia cuatro tratamientos de luz (AB las hojas fueron expandidas bajo el sol y fueron transferidas a la sombra,BA las hojas experimentaron la transferencia contraria,y las hojas controles AA y BB que fueron expandidas y mantenidas bajo condiciones de sol o de sombra).Las hojas adultas o jóvenes no difieren en la eficiencia fotoquímica general.Por el contrario,se encontró diferencias entre los ambientes de luz (iluminados), para los cuales las hojas transferidas de la sombra al sol mostraron las menores tasas Fv /Fm .No hubo relación entre la eficiencia fotoquímica y la temperatura de las hojas. En P.limonensis ,las plantas son suceptibles a la foto -inhibición independientemente del estado ontogenético de la hoja. El enfoque utilizado en este experimento permitió la rápida exploración de esta capacidad y demostró que puede ser utilizado en otras especies poco estudiadas del sotobosque.
Palabras clave :fluorescencia,clorofila,fotoinhibición,fotosíntesis,Psychotria limonensis ,bosque tropical,Panamá.
References
Araus,J.L.&K.P.Hogan.1994.Leaf structure and patterns of photoinhibition in two neotropical palms in clearings and forest understory during the dry season.Am. J.Bot.81:726-738. [ Links ]
Avalos,G.1999.Photosynthetic acclimation of canopy branches and seedlings of lianas to light changes in a tropical dry forest.Ph.D.Thesis.University of Missouri-St.Louis,St.Louis Missouri. [ Links ]
Avalos,G.&S.S.Mulkey.1999.Photosynthetic acclimation of the liana Stigmaphyllon lindenianum to light changes in a tropical dry forest.Oecologia 120: 475-484. [ Links ]
Barone,J.A.1998.Effects of light availability and rainfall on leaf production in a moist tropical forest in central Panama. J.Trop.Ecol.14:309-321. [ Links ]
Bauer,H.&W.Thöni.1988.Photosynthetic light acclimation in fully developed leaves of the juvenile and adult life phases of Hedera helix .Physiol.Plant.73: 31-37. [ Links ]
Bazzaz,F.A.&R.W.Carlson.1982.Photosynthetic acclimation to variability in the light environment of early and late successional plants.Oecologia 54:313-316. [ Links ]
Chazdon,R.L.&N.Fetcher.1984.Photosynthetic light environments in a lowland tropical rain forest in Costa Rica.J.Ecol.72:553-564. [ Links ]
Chazdon,R.L.,R.W.Pearcy,D.W.Lee &N.Fetcher. 1996.Photosynthetic responses of tropical forest plants to contrasting light environments.p.5-55. In S.S.Mulkey,R.L.Chazdon &A.P.Smith (eds.). Tropical Forest Plant Ecophysiology.Chapman & Hall,New York. [ Links ]
Croat,T.B.1978.Flora of Barro Colorado Island.Stanford University Press,Stanford,CA. [ Links ]
Demmig,B.&O.Björkman.1987.Comparison of the effect of excessive light on chlorophyll fluorescence (77 K)and photon yield of O2 evolution in leaves of higher plants.Planta 71:171-184. [ Links ]
Denslow,J.S.1980.Gap Partitioning among Tropical Rainforest Trees.Biotropica 12:47-55. [ Links ]
Denslow,J.S.1987.Tropical rainforest gaps and tree species diversity.Ann.Rev.Ecol.Syst.18:432-451. [ Links ]
Dickson,R.E.&J.G.Isebrands.1991.Leaves as regulators of stress response.p.3-34.In H.A.Mooney,W.E. Winner,E.J.Pell &E.Chu (eds.).Response of Plants to Multiple Stresses.Academic,New York. [ Links ]
Fernández,D.&N.Fetcher.1991.Chlorophyll fluorescence and quantum yield in seedlings and saplings of tropical forest.Primer Congreso Venezolano de Ecología.Resúmenes,Sociedad Venezolana de Ecología,Caracas. [ Links ]
Fetcher,N.,S.F.Oberbauer &R.L.Chazdon.1994. Physiological Ecology of Plants.p.128-141.In L.A. McDade, K.S. Bawa, H.A.Hespenheide &G.S. Hartshorn (eds.).La Selva:Ecology and Natural History of a Neotropical Rain Forest.University of Chicago,Chicago. [ Links ]
Field,C.B.1991.Ecological Scaling of Carbon Gain to Stress and Resource Availability.p.35-63.In H.A. Mooney,W.E.Winner &E.J.Pell (eds.).Response of Plants to Multiple Stresses.Academic,New York. [ Links ]
Grubb,P.J.1977.The maintenance of species richness in plant communities:the importance of the regeneration niche.Biol.Rev.52:107-145. [ Links ]
Gouallec,J.L.L.,G.Cornic &P.Blanc.1990.Relations between sunfleck sequences and photoinhibition of photosynthesis in a tropical rain forest understory herb.Amer.J.Bot.77:999-1006. [ Links ]
Gouallec,J.L.L.,G.Cornic &J.M.Briantais.1991. Chlorophyll fluorescence and photoinhibition in a tropical rainforest understory plant.Photosynthesis Res.27:135-142. [ Links ]
Kitajima,K.,S.S.Mulkey &S.J.Wright.1997.Seasonal leaf phenotypes in the canopy of tropical dry forest: photosynthetic characteristics and associated traits. Oecologia 109:409-498. [ Links ]
Krause,G.H.1988.Photoinhibition of photosynthesis.An evaluation of damaging and protective mechanisms. Physiol. Plantarum 74:566-574. [ Links ]
Krause,G.H.,A.Virgo &K.Winter.1995.High susceptibility to photoinhibition of young leaves of tropical forest trees.Planta 197:583-591. [ Links ]
Long,S.P.,S.Humphries &P.G.Falkowski.1994. Photoinhibition of photosynthesis in nature.Annu. Rev.Plant Physiol.Mol.Biol.45:633-662. [ Links ]
Lovelock,C.E,M.Jebb &C.B.Osmond.1994. Photoinhibition and recovery in tropical plant species: response to disturbance.Oecologia 97:297-307. [ Links ]
Mulkey,S.S.&R.W.Pearcy.1992.Interactions between acclimation and photoinhibition of photosynthesis of a tropical forest understory herb:Alocasia macrorrhiza ,during simulated canopy gap formation. Functional Ecol.6:719-729. [ Links ]
Osmond,C.B.1981.Photorespiration and photoinhibition: some implications for the energetics of photosynthesis. Biochim.Biophys Acta.639:77-98. [ Links ]
Wright,S.J.,J.L.Machado,S.S.Mulkey &A.P.Smith. 1992.Drought acclimation among tropical forest shrubs (Psychotria ,Rubiaceae).Oecologia 89: 457-463. [ Links ]
Wright ,S.J.1996.Phenological responses to sasonality in tropical forest plants.p.440-60.In S.S.Mulkey, R.Chazdon &A.Smith (eds).Tropical Forest Plan Ecophysiology.Chapman &Hall,New York. [ Links ]












 uBio
uBio