Revista de Biología Tropical
versión On-line ISSN 0034-7744versión impresa ISSN 0034-7744
Rev. biol. trop vol.52 no.3 San José sep. 2004
Genetic diversity of Costa Rican populations of the rice planthopper Tagosodes orizicolus (Homoptera: Delphacidae)
Myriam Hernández1 , Tania Quesada1 , Claudia Muñoz1 & Ana M. Espinoza1,2
1 Centro de Investigación en Biología Celular y Molecular (CIBCM), Ciudad de la Investigación, Universidad de Costa Rica, Sabanilla de Montes de Oca, San José, Costa Rica.
2 Escuela de Agronomía, Facultad de Ciencias Agroalimentarias, Universidad de Costa Rica, San Pedro, San José, Costa Rica.
Received 20-VIII-2002. Corrected 09-VI-2003. Accepted 30-VII-2003.
Abstract
Tagosodes orizicolus (Homoptera: Delphacidae) is one of the main constraints of the rice production in the Neotropics. This planthopper produces severe damages as a phloem feeder, causes mechanical injury during oviposition and vectors the rice hoja blanca virus (RHBV). The main objective of this study was to determine the genetic diversity of T. orizicolus populations from three rice growing regions of Costa Rica, using RAPDs. Individuals from Guanacaste, Parrita, San Carlos and Cali-Colombia, as outgroup, were analyzed using the random primers. Phenetic relationships revealed that the Costa Rican populations were clearly separated from Cali-Colombia, sharing less than 25% similarity. Costa Rican populations were divided into two main branches separated at 30% similarity. The first branch included Guanacaste and San Carlos and the second displayed Parrita. In relation to similarity indexes within groups, the Guanacaste cluster showed the highest (over 50%) and Cali-Colombia was the most diverse (28%). The correspondence analysis confirmed the clusters of the phenogram and showed close interactions between the Parrita and San Carlos populations. The genetic separation observed could be the result of the geographic isolation among populations, but it could also be explained by the infection with the rickettsia Wolbachia pipientis. This bacterium causes cytoplasmic incompatibility in its host, which results in non-viable progeny when infected males mate with non-infected females, or when insects hosting different strains of Wolbachia mate. Then, a search for Wolbachia in previously described populations of T. orizicolus was initiated. The presence of the bacteria was analyzed by PCR with 16S rDNA-specific primers for Wolbachia. The PCR analyses revealed infections of 86% in the population of San Carlos, 96% in Guanacaste, 37% in Parrita and 100% in Cali-Colombia. Crosses between individuals of T. orizicolus from Parrita and Guanacaste were performed for testing cytoplasmic incompatibility. When infected males were crossed with non-infected females within the same population, a significant reduction in progeny number was obtained as well as when crosses between infected individuals belonging to different populations were performed. These experiments showed cytoplasmic incompatibility not only caused by the presence of Wolbachia within the population, but also by the presence of different strains of the bacteria between populations. Rev. Biol. Trop. 52(3): 795-806. Epub 2004 Dic 15.
Key words: Tagosodes orizicolus, delphacid, genetic diversity, Wolbachia, RAPDs, cytoplasmic incompatibility.
Palabras clave: Tagosodes orizicolus, delfácido, diversitdad genética, Wolbachia, RAPDs, incompatibilidad citoplasmática.
The planthopper Tagosodes orizicolus Muir (Homoptera: Delphacidae) is one of the main constrains in many rice growing areas of the Neotropics. The effect of this monophagous rice insect can be very severe on rice plantations, as its repeated feeding and oviposition, combined with high population densities, can cause severe mechanical damage to the plant, thus reducing rice production. T. orizicolus is also the vector and host of the rice hoja blanca virus, which is one of the main rice diseases of tropical America (Reynaud 1963, Galvez 1968). This insect presents sexual dimorphism and is recognized by a spur in the rear tibia (characteristic of the family) and by the morphology of the male genitalia (Wilson and Claridge 1991). Brachypterous and macropterous forms are commonly found in females, and it is believed that the relative abundance of these forms is due to population dynamics, environment-linked characters and the physiological stage of the host plant (Denno and Perfect 1994). In addition, it is known that delphacid macropterous forms profit from wind currents to travel long distances and colonize new fields, hence favoring insect dispersal (Kisimoto and Rosenberg 1994). In contrast, brachypterous forms are more cryptic in habit and have a higher number of progeny than the highly dispersive macropterous forms (Perfect and Cook 1994).
In Costa Rica, the main rice-producing areas are geographically separated and have very different climatic conditions. Generally, rice is cultivated under a rainfed system, as it occurs in the Central and South Pacific regions, as well as in the Northern part of the country. In the North Pacific, rice is cultivated both under rainfed and flooded systems. T. orizicolus is present in all rice producing areas; however, the geographical isolation of these areas and the differences in climate and in rice planting systems could affect the genetic structure of the populations. On the other hand, wind currents could provide conditions for insect migration among the rice-growing areas.
Although in Asia delphacid long-distance migration is well documented for Nilaparvata lugens and Sogatella furcifera, there are no data on migration of the rice planthopper Tagosodes orizicolus in the Neotropics. In Costa Rica, the strong, predominant Northeastern trade winds reach the Caribbean coast, where they are slowed down by the mountain ranges that separate the Caribbean from Pacific slopes. The more seasonal Western equatorial and synoptic winds from the Pacific Ocean hit the North Pacific lowland regions of the country, and are intercepted by the mountain ranges (Zárate 1977, 1978, Ulate 1994). Ocean breezes predominantly influence the Central and South Pacific lowlands and rarely flow past the mountain ranges of Tilarán and Talamanca into the West. Low depressions in the Guanacaste mountain range, however, allow the strong wind currents of the trade winds to flow from the Northern plains to the North Pacific region, possibly resulting in occasional insect migration (Alvarado, personal communication).
In addition, the bacteria Wolbachia pipientis has been known to influence the genetic structure of insect populations because it induces cytoplasmic incompatibility, both unidirectional and bidirectional (Hertig 1936, Laven 1959, Smith-White and Woodhill 1954). Wolbachia infections have been detected in some arthropods and many insect species, including Drosophila simulans (Laven 1959), Aedes scutellaris (Smith-White and Woodhill 1954) and in the delphacid Laodelphax striatellus (Noda 1984). Unidirectional incompatibility caused by this bacterium occurs when infected males mate with non-infected females, making the eggs die from failure in chromatin condensation, as a product of incomplete karyogamy (Noda 1984, Boyle et al. 1993, Werren and Jaenike 1995). In contrast, infected females can mate with both infected and non-infected males, which suggests that the effects of Wolbachia during sperm development can be neutralized when bacteria are present in the egg. Therefore, infected females exhibit a reproductive advantage within a mixed population of infected and non-infected individuals (Noda 1987, Werren et al. 1995). Bidirectional incompatibility is caused by different strains of Wolbachia, where insects hosting different strains of the bacteria fail to reproduce.
The hypothesis, therefore, is that well-defined groups may exist in the rice-growing areas, although some occasional migration events may occur. The purpose of this study was to analyze the genetic diversity of Tagosodes orizicolus populations from three different rice producing regions in Costa Rica using DNA polymorphisms determined by RAPD-PCR. It also intended to detect Wolbachia in natural populations of T. orizicolus by means of the amplification of partial 16S ribosomal DNA sequences. Additionally, it was intended to detect cytoplasmic incompatibility by means of crossing infected and non-infected individuals from two geographically isolated populations.
Material and methods
Collection and colony establishment of T. orizicolus: Insects for genetic analyses were collected from the geographically separated locations of Parrita (N 09°3415.3"; W 084°3323.3"), Guanacaste (N 10°2904.9"; W 085°2423.3") and San Carlos (N 10°2506.5"; W84°3051.7"). A suction motor pump (CRAFTZMAN 32cc, USA) was used to collect the insects into a fine mesh cloth placed between two extensions of the suction duct. These were later transferred on ice to the laboratory and were stored at 4°C prior to their selection and processing. Individuals of T. orizicolus were separated from other insect species using a dissecting microscope. Insects with malformations or external parasitoids were discarded.
In addition, insect DNA donated by the Centro Internacional de Agricultura Tropical (CIAT), from Cali, Colombia, was analyzed as an outgroup. For the crossing experiments, insects from the populations of Parrita andGuanacaste were collected with the suction pump, transferred to mesh-covered cages and transported to the insectarium, where colonies of T. orizicolus were established.
Genomic DNA extractions: DNA was extracted according to the procedures of Hunt and Page (1992), Williams et al. (1992) and Landry et al. (1993). The selected insects were placed individually in microcentrifuge tubes with homogenizing buffer (1% CTAB buffer, 0.75 M NaCl, 50 mM Tris-HCl pH 8, 10 mM EDTA and 100 µg/ml DNAse-free proteinase K). The samples were homogenized, incubated for two hours at 60°C, and centrifuged. The supernatant was transferred to a clean tube, and treated twice using 1:1 phenol:chloroform. DNAwas precipitated, resuspended in distilled water (SIGMA W3500) and treated with RNAse (20 mg/ml). DNA concentration was estimated by spectrophotometry at 260 nm (Sambrook et al. 1989) and samples were stored at -30°C.
Molecular analyses using RAPD-PCR: Thirty insects per population were analyzed using RAPDs. DNA was amplified in a Perkin Elmer Cetus 2400 thermal cycler. For the amplifications, five random primers with a length of ten nucleotides were used (Operon Technologies, Inc: OPA-07, OPA-04, OPA-11, OPA-18 and OPA-16) (Table 1). The conditions for each reaction were as follows: 1X PCR buffer, 2.5 mM MgCl 2 , 0.1 mM dNTPs, 0.025 U/µl Taq DNA polymerase (Perkin Elmer, New England Biolabs), 0.2 µM primers and 2 ng/µl of insect genomic DNA. The samples were amplified for 40 cycles: 30 seconds at 94°C, 30 seconds at 35°C and 60 seconds at 72°C. PCR products were analyzed in 1.5% agarose gels stained with ethidium bromide. Bands were observed under UV light and pictures were taken with 677 Polaroid film.
Statistical analyses: In order to determinate the phenetic relationships of the samples, the presence and absence of DNA bands were given values of 1 and 0, respectively, and were converted into a binary matrix. Only the bands located in the middle section of the gel were recorded (bands sizes from 4 Kb to 220 bp). The genetic similarity among populations was determined using the Dice Similarity Coefficient and the SIMQUAL program from the "Numerical Taxonomy and Multivariate Analysis System" (NTSYS pc2.1). The cluster analysis was performed using the Unweighed Pair Group Method with Arithmetic Mean (UPGMA) from SAHN program to obtain the phenogram. Furthermore, a correspondence analysis was carried out with NTSYSs pc2.1. Finally, a bootstrap analysis was performed using the TREECON 1.3b program for windows and 1000 resamples.
Transmission electron microscopy: The head and thorax of males and females of T. orizicolus were removed and the abdomens were fixed in 2% paraformaldehyde and 2.5% glutaraldehyde in a 0.1 M sodium phosphate solution, pH 7.2 (Karnovsky 1965, Kozuka 1989). Air was extracted from the samples with a hypodermic needle and fixation was performed at 4°C during 12 hours, followed by repeated washing with 0.1 M phosphate buffer, pH 7.2. Samples were then post-fixed in 2% osmium tetraoxide for two hours at room temperature and washed with phosphate buffer. These were later dehydrated in an ethanol gradient, which was gradually substituted by propylene oxide. Tissues were infiltrated in Spurr resin, soaked in flat dishes to facilitate sample orientation and the resin polymerization was performed at 60°C for 72 hours. Ultra thin sections were obtained using an MT2-B ultramicrotome, collected in 200 mesh copper grids and stained with 4% uranyl acetate. These were then washed with 50% ethanol and contrasted with lead citrate. The grids were then washed several times with distilled water and observed in a transmission electron microscope (Hitachi H-700) at 3000 to 40000 X amplification.
Detection of Wolbachia by PCR: DNA from twenty-five insects of Guanacaste, Parrita, San Carlos and Colombia were analyzed using specific primers to detect the presence of Wolbachia. Total DNA quality was determined using primers that amplify specific insect mitochondrial 12S rDNA sequences, known as 12SAI (5´-AAACATGGATTAGAT-ACCCTATTAT) and 12SBI (5´-AAGAGC- ACGGGCGATGTGT). Positive samples were then analyzed with the Wolbachia 16S ribosomal DNA primers (5´-TTGTAGCCT-GCTATGGTATAACT and 5´-GAATAG-GTATGATTTTCATGT) (ONeill et al. 1992). The primers were provided by Dr. Scott ONeill from Yale University, USA. DNA amplification was performed according to the following program: an initial phase of 90 seconds at 95ºC, followed by 30 cycles (30 s at 95°C, 30 s at 55°C and 60 s at 72°C). PCR products were analyzed in 1% agarose gels.
Crossing experiments: A total of 30 nymphs of the fourth and fifth instars of Parrita and Guanacaste populations were individually confined in plastic tubes on 30-day-old rice plants. When the nymphs reached the adult stage, a male and a female from each population were placed in plastic tubes on rice plants for mating and oviposition. In addition, crosses of male and females from a same population were performed under the same conditions described above. One week later, adults were collected and sacrificed for DNA extraction for a PCR-based survey of Wolbachia. Two weeks later, plants were examined to determine the number of offspring resulting from each cross.
Results
Of the 120 T. orizicolus insects analyzed, 76 amplified with all the primers evaluated. Between 10 and 23 bands were obtained for each primer used, resulting in a total of 72 bands. The primer OPA-11 was the most polymorphic, showing a total of 23 different bands. The primers used were the most polymorphic obtained from Operon® RAPD primers that had previously amplified for aphids (Williams et al. 1992), as no previous information on RAPD analyses of T. orizicolus had been previously reported. These bands showed molecular weights that ranged from 4Kb to 200 bp (Table 1).
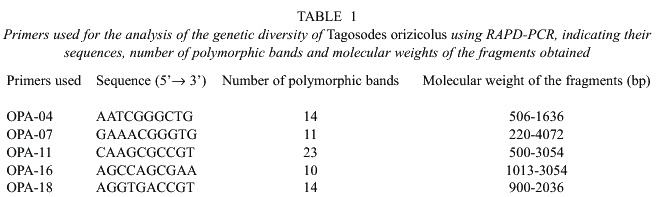
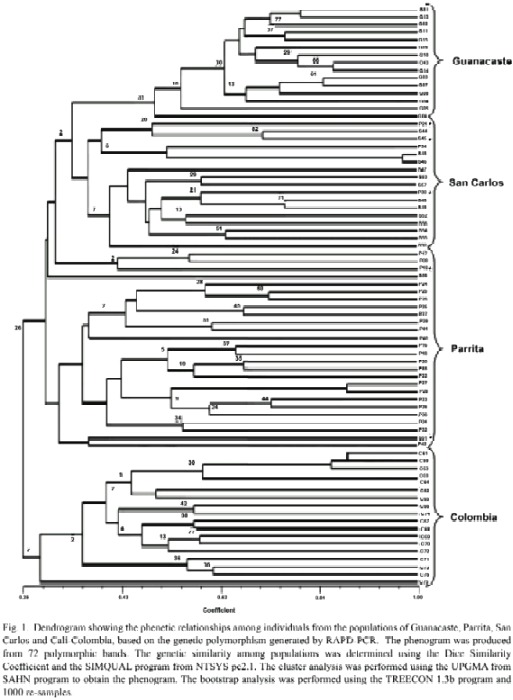
For the statistical analyses, a binary matrix was obtained and a dendrogram of the phenetic relationships among the T. orizicolus populations from Guanacaste, Parrita and San Carlos was constructed (Fig. 1). Four clusters that grouped individuals of T. orizicolus according to the geographical sites from which they were collected were observed: Guanacaste, Parrita, San Carlos and Cali-Colombia. However some exceptions were detected in a few individuals where two specimens from San Carlos grouped with the Parrita cluster and three from Parrita grouped with the San Carlos cluster (Fig. 1). The three Costa Rican populations were clearly separated from the Cali-Colombia cluster, sharing less than 25% similarity. Populations from Costa Rica formed two main branches that separated at 30% similarity, the first included Guanacaste and San Carlos populations and the second displayed the Parrita cluster. In relation to similarity indexes within groups, the Guanacaste cluster showed the highest (over 50%), Parrita and San Carlos had less than 32%, and Cali-Colombia was the most diverse (28%) (Fig. 1). The bootstrap analysis showed low indexes for many branches, suggesting a high variation within the four populations of T. orizicolus (Fig. 1). The correspondence analysis confirmed the clusters observed in the phenogram, and showed a clear separation of the Cali-Colombia and Guanacaste clusters from Parrita and San Carlos (Fig. 2). At the same time it showed close interactions between the Parrita and San Carlos populations. In addition, the analysis also depicted that the individuals from Guanacaste constitute a compact group while the Cali-Colombia and the Parrita clusters are less compact (more diverse).
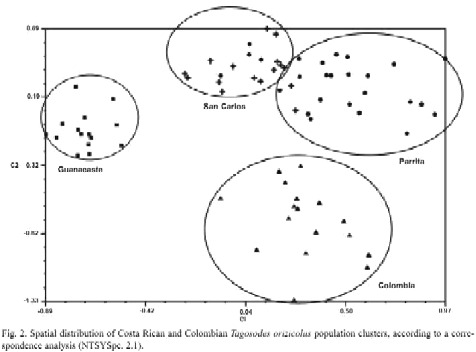
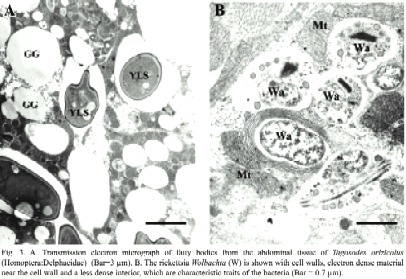
The detection of Wolbachia through PCR analyses revealed different infection percentages among the populations studied. The Colombian individuals displayed 100% infection, whereas Parrita, Guanacaste and San Carlos showed infections of 37%, 96% and 86%, respectively. In situ detection of Wolbachia in the insects abdomen revealed its presence mainly in fat tissues, both in the form of isolated bacteria or in small groups. Wolbachia presented typical characteristics of the rickettsia group, such as pleomorphism, rigid cell wall, electron-dense material associated with the cell wall and a less dense interior (Fig. 3a). Its size in transverse sections was ca. 520 nm long and 470 nm wide (Fig. 3b). Yeast-like symbiotes (YLS) were also abundant in the same tissues, as previously reported by Espinoza et al. (2004).
Cytoplasmic incompatibility experiments performed by crossing individuals from Guanacaste and Parrita, revealed that when infected males and females from the same population were crossed, no incompatibility was observed (Crosses 1 and 5, Table 2).
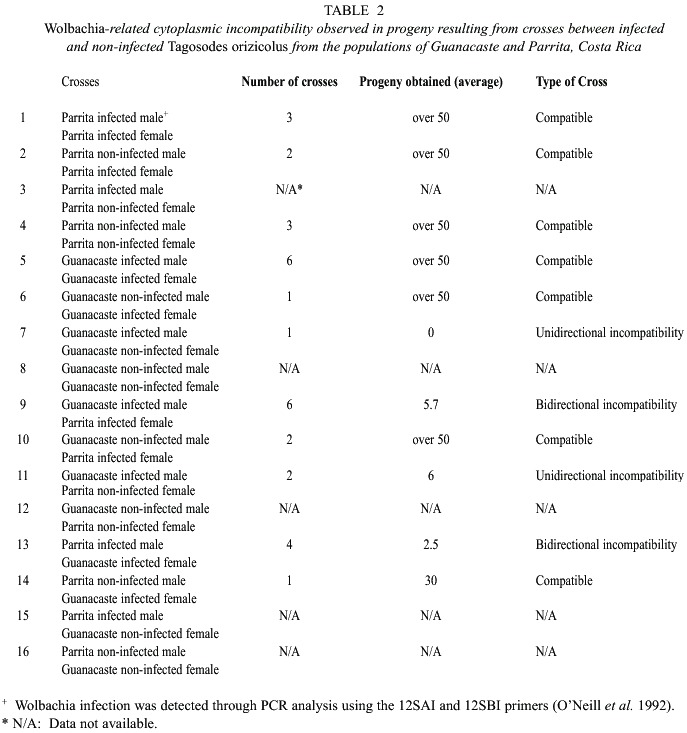
However, when infected males and females from different populations were crossed, a dramatic reduction in progeny number was registered suggesting bidirectional incompatibility (Cross 9 and 13, Table 2). Furthermore, abundant progeny (more than fifty individuals) was obtained from crosses between infected females and non-infected males (Crosses 2, 6, 10 and 14, Table 2), regardless of whether the insects belonged to the same or different populations. When infected males mated with non-infected females, few or no progeny were observed (Crosses 7, and 11, Table 2) whereas crosses between non-infected males and females produced abundant progeny (Cross 4, Table 2). As observed in Table 2, some crosses did not occur, because Wolbachia infection could not be detected prior to mating.
Discussion
Amplification products obtained were highly polymorphic, resulting in highly variable patterns for each individual, as observed for OPA-11, which displayed the most polymorphic bands. Given the high polymorphism observed, the five primers used were sufficient to revealed a clear clustering among individuals from the four populations analyzed. The Colombian population was included as an out-group, and it separated clearly from Costa Rican individuals, sharing similarity percentages of approximately 25%. This could be explained by the geographical separation between Costa Rica and Colombia, where physical distance, mountain ranges and dense forests could prevent insect migration. Clear separation among the Costa Rican populations was also observed, producing three well-defined clusters. This suggests that gene flow among populations is not a common event. It has been observed that in temperate regions insect migration is predominantly seasonal and covers long distances, whereas in the tropics migration typically occurs over distances that range between 5-30 km (Perfect and Cook 1994). In addition, monophagous species are more likely to migrate and track changes in the quality of their host plant. However, if the crop is grown year-round, as occurs in irrigated systems in the tropics, the immediate food availability may cause insects to migrate for short distances between fields.
Based on the physical map of Costa Rica, it was postulated that insect migration from San Carlos to Guanacaste could occur. However, the Guanacaste volcanic range constitutes an important physical barrier for insect migration, although sporadic events could occur under the influence of the trade winds flowing from east to west (Alvarado, personal communication). The results obtained through RAPD-PCR analyses suggest that the Guanacaste mountain range was an effective barrier between these two populations, as no individuals from Guanacaste grouped in the San Carlos cluster (Fig. 1). Nevertheless, future studies that utilize larger sample sizes or populations in different locations between the two regions, may detect migration events between these localities.
It is interesting to notice that three individuals from Parrita grouped with the San Carlos population and two from San Carlos grouped with the Parrita cluster, suggesting gene flow between these two populations. It is interesting to mention that the correspondence analysis showed individuals from both populations that had close similarities. It is difficult to explain migration events from Parrita to San Carlos, as the trade winds flow predominantly in the opposite direction (Zárate 1977, 1978). It has been observed that delphacids can actively fly up to 30 km in the tropics (Perfect and Cook 1994). The distances traveled are then determined by the wind currents in which the insects are flying, as well as by the amount of time they can remain airborne through wing flapping and gliding on updrafts (Kisimoto and Rosenberg 1994).
The intrapopulation similarity indexes within the Costa Rican populations varied between 30-50%, although environmental conditions and agricultural practices are different among the three populations. The region of Guanacaste has produced rice year-round for over 20 years, with overlapping plantations. This allows the insects a constant food source throughout the year. In contrast, the insect populations of Parrita and San Carlos face very different conditions, mainly because rice is produced only during the rainy season (June to December), which means that during the dry season (about four months) the insects cannot tap into their main food source. Consequently, these insect populations are dramatically reduced during the dry season (Oliva 1998), a fact that possibly causes a population bottle-neck after each season. Insect survival during the dry season is still unknown, as no alternative hosts for T. orizicolus have been identified in Parrita, except volunteer rice. Migration, diapause or alternative hosts may represent some survival mechanisms for this species, although the latter have been more widely studied in higher latitudes (Kisimoto and Rosenberg 1994). In previous studies using alternative Poaceae hosts for T. orizicolus under greenhouse conditions revealed that this insect could only complete its life cycle on Oryza sativa (Oliva 1998). However, in further experiments, T. orizicolus has been found to complete its life cycle under greenhouse conditions on Oryza glumaepatula (unpublished results), a wild relative of rice found in a population restricted to two wetlands in the northern part of the country (Los Chiles and Murciélago) (Zamora 2001). Based on these results, it is unlikely that this species represents an important alternative host to T. orizicolus in Parrita, especially since O. glumaepatula has not been found in the Pacific lowlands, nor associated with rice fields.
Genetic separation among Costa Rican populations of T. orizicolus could also be explained by their infections with different strains of Wolbachia. Different levels of bacterial infection were observed among the populations studied, of which Guanacaste showed the highest levels of infection (96%). A possible explanation for the above result could be that the continuous rice-planting cycles in this area allow for a greater number of insect generations per year, hence increasing the number of infected individuals. Since Wolbachia is transmitted vertically, and therefore related to the insect reproduction, this agricultural practice may favor its rapid spread within the population. In contrast, the lower levels observed in Parrita (37%), could be due to the fact that only one cycle is planted per year during the rainy season. It would therefore be interesting to monitor Wolbachia infection through different periods to determine changes in its incidence over time. At the same time, it would be interesting to monitor both the genetic diversity of T. orizocolus and the incidence of Wolbachia through time to see if this bacteria could influence the populations structure of T. orizicolus.
The crossing experiments between individuals of Guanacaste and Parrita showed that Wolbachia caused unidirectional and bidirectional cytoplasmic incompatibility (CI), which suggests the presence of at least two different strains of the bacteria in Costa Rica. However, some crossing events did not occur, as infected or non-infected individuals could only be detected by sacrificing the parents after mating and oviposition took place. The high levels of Wolbachia infection in Guanacaste (96%) could be a reason for the difficulty of mating between non-infected individuals (see Cross 8, Table 2). This was also reflected in the lack of results observed in the interpopulation crosses between infected Guanacaste and Parrita individuals, due to low infection levels in Parrita (Crosses 12, 15 and 16, Table 2). In contrast, crosses involving infected individuals from Guanacaste were more abundant (Crosses 5 and 9, Table 2). In addition, the low infection levels of Parrita with Wolbachia (37%), resulted in crosses between non-infected individuals (Cross 4, Table 2), whereas the lack of data observed in cross 3 (Table 2) could be mainly due to sample size rather than to infection percentages. This information on cytoplasmic incompatibility has been very useful for establishing insect colonies in laboratory conditions, since mixing insects from different populations could cause a reproductive failure of the colony. In summary, geographical barriers and cytoplasmic incompatibility due to Wolbachia seem to be influencing the genetic structure of T. orizicolus populations in Costa Rica.
Acknowledgments
The authors wish to thank Scott ONeill, from Yale University, for providing the primers used in Wolbachia detection, Myriam Cristina Duque, from CIAT, and Federico Albertazzi for the critical review of this manuscript.
Resumen
Tagosodes orizicolus (Homoptera: Delphacidae) es uno de las principales plagas de arroz en el Neotrópico, ya que se alimenta de la savia del floema, realiza incisiones durante la oviposición que dañan los haces vasculares de las hojas y es el vector del virus de la hoja blanca del arroz (RHBV). El objetivo de esta investigación es determinar la diversidad genética de las poblaciones de T. orizicolus de las principales zonas arroceras del Costa Rica. Para ello se analizaron mediante RAPDs individuos de las población de Guanacaste, Parrita y San Carlos y como grupo externo Cali-Colombia. El fenograma mostró que los individuos agruparon de acuerdo a su localidad, observándose que las tres poblaciones costarricenses se separaron de la Colombiana, compartiendo menos del 25% de similaridad. Las poblaciones costarricenses se separaron en dos brazos principales a un 30% de similaridad: el primero incluyó Guanacaste y San Carlos, mientras que el segundo lo constituyó Parrita. El conglomerado de Guanacaste mostró un índice de similaridad del 50% entre individuos mientras que la población de Cali-Colombia fue la más diversa (28%). Finalmente, el análisis de correspondencia confirmó los conglomerados observados en el fenograma y mostró una interacción cercana entre las poblaciones de Parrita y San Carlos. La separación genética observada podría ser el resultado del aislamiento geográfico entre poblaciones pero también podría explicarse por la infección con la rickettsia Wolbachia pipientis. Esta bacteria causa incompatibilidad citoplasmática en sus hospederos, lo cual resulta en una progenie no viable en los cruces entre machos infectados con hembras no infectadas, o cuando ocurren cruces entre insectos infectados con diferentes cepas de Wolbachia. Otro objetivo de esta invsetigación fue determinar por PCR la presencia de Wolbachia en las poblaciones antes mencionadas, utilizando iniciadores específicos para el rADN 16S. Dicho análisis reveló infecciones del 86% en la población de San Carlos, un 96% en Guanacaste, un 37% en Parrita y un 100% en Cali-Colombia. Con el fin de determinar si se presenta incompatibilidad citoplasmática, se llevaron a cabo cruces entre individuos de T. orizicolus de Parrita y Guanacaste. Se observó una reducción significativa en la progenie en los cruces entre machos infectados y hembras no infectadas de una misma población, así como, en los cruces entre individuos infectados pertenecientes a poblaciones diferentes. Estos resultados mostraron incompatibilidad citoplasmática, no sólo causada por la presencia de Wolbachia, sino también por la presencia de diferentes cepas de la bacteria entre poblaciones de T. orizicolus.
References
Bordenstein, S.R., F.P. OHara & J.H. Werren. 2001. Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature 409: 707-710. [ Links ]
Boyle, L., S. ONeill, M.H. Robertson & T.L. Karr. 1993. Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260: 1796-1799. [ Links ]
Denno, F.R. & J.T. Perfect. 1994. Planthoppers as models for ecological study and effective pest management. In F.R. Denno & T.J. Perfect (eds.). Planthoppers: Their ecology and management. Chapman & Hall. pp. 1-4. [ Links ]
Espinoza, A.M., A.M. Xet-Mull, R. Mora & E. Sánchez. 2004. Morphologic characterization of yeast-like symbiotes of Tagosodes orizicolus (Homoptera: Delphacidae) by light and electron microscopy. Rev. Biol. Trop. 52: in press. [ Links ]
Galvez, G.E. 1968. Transmission studies of the rice hoja blanca virus with highly active, virus-free colonies of Sogatodes oryzicola. Disease Reporter 45: 949-953. [ Links ]
Gaspari, G., R.A. Malacrida, C. Bandi, R.C. Guglielmino, G. Damiani & L. Baruffi. 1995. Polymorphism within and between populations of Ceratitis capitata: comparison between RAPD and multilocus enzyme electrophoresis data. Heredity 74: 425-437. [ Links ]
Hertig, M. 1936. The rickettsia, Wolbachia pipientis (gen. et. sp. n.) and associated inclusions of the mosquito Culex pipiens. Parasitology 28: 453-486. [ Links ]
Hunt, J.G. & E.R. Page. 1992. Patterns of inheritance with RAPD molecular markers reveal novel types of polymorphism in the honeybee. Theoretical and Applied Genet. 85: 15-20. [ Links ]
Janzen, D.H. 1993. Caterpillar seasonality in a Costa Rican dry forest. pp. 448-477. In N.E. Stamp & T.E. Casey (eds.). Caterpillars. Ecological and evolutionary constraints on foraging. Chapman & Hall, New York. [ Links ]
Joint Plant Breeding Symposia Series. 1992. Applications of RAPD technology to plant breeding. Crop Science Society of America, American Society for Horticultural Science, American Genetic Association. 52 p. [ Links ]
Karnovsky, M.J. 1965. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J. Cell Biol. 27: 137. [ Links ]
Kisimoto, R. & J. Rosenberg, 1994. Long-distance migration in delphacid planthoppers. In F.R. Denno & T.J. Perfect (eds.). Planthoppers: Their ecology and management. Chapman & Hall. pp. 302-322. [ Links ]
Kozuka, Y. 1989. Laboratory manual for transmission electron microscope specimen preparation. Centro de Investigación en Biología Celular y Molecular CIBCM, Universidad de Costa Rica. [ Links ]
Landry, B.S., L. Dextraze & G. Boivin. 1993. Random amplified polymorphic DNA markers for DNA fingerprinting and genetic variability assessment of minute parasitic wasp species (Hymenoptera: Mymaridae and Trichogrammatidae) use in biological control programs of phytophagous insects. Genome 36: 580-587. [ Links ]
Laven, H. 1959. Speciation by cytoplasmic isolation in the Culex pipiens complex. Cold Spring Harbor Symposia in Quantitative Biology 24: 166-175. [ Links ]
Morales, F.J. & A.I. Niessen. 1985. Rice hoja blanca virus. Description of plant viruses, N° 299. Commonwealth Mycological Institute/Association of Applied Biologists. [ Links ]
Noda, H. 1984. Cytoplasmic incompatibility in allopatric field populations of the small brown planthopper Laodelphax striatellus, in Japan. Entomol. Exp. Appl. 35: 263-267. [ Links ]
Noda, H. 1987. Further studies of cytoplasmic incompatibility in local populations of Laodelphax striatellus in Japan (Homoptera: Delphacidae). Applied Entomol. Zool. 4: 443-448. [ Links ]
Oliva, M. 1998. Fluctuación poblacional de delfácidos y su ámbito de poáceas hospederas asociadas al arroz en Liberia, Guanacaste. Tesis Lic. en Ingeniería Agronómica, Universidad Nacional, Heredia, Costa Rica. [ Links ]
ONeill, S.L., R. Giordano, A.M.E. Colbert, T.L.A. Karr & H.M. Robertson. 1992. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Nat. Acad. Sci. U.S.A. 89: 2699-2702. [ Links ]
Perfect, T.J. & A.G. Cook. 1994. Rice planthopper population dynamics: A comparison between temperate and tropical regions. In F.R. Denno & T.J. Perfect (eds.). Planthoppers: Their ecology and management. Chapman & Hall. pp. 282-301. [ Links ]
Reynaud, G.G. 1963. Biología, ecología, combate y pruebas de transmisión con Sogatoda orizicola Muir y Sogata cubana (Crauf)- (Araepodidae-Homoptera), vectores del virus de la "Hoja Blanca" del arroz. Tesis de la Escuela Nacional de Agricultura, Chapingo, México. 50 p. [ Links ]
Sambrook, J., T. Maniatis & E.F. Fritsh. 1989. Molecular cloning; a laboratory manual. New York, Cold Spring Harbor Laboratory. [ Links ]
Smith-White, S., A.R. Woodhill. 1954. The nature and significance of non-reciprocal fertility in Aedes scutellaris and other mosquitoes. Proc. Linnaean Soc. New South Wales 79: 163-176. [ Links ]
Ulate, V.G. 1994. El clima de Costa Rica: contraste de dos vertientes. San José, Costa Rica. Editorial Guayacán. 58 p. [ Links ]
Werren, J.H. & J. Jaenike. 1995. Wolbachia and cytoplasmic incompatibility in micophagous Drosophila and their relatives. Heredity 75: 320-326. [ Links ]
Werren, J.H., W. Zhang & L.R. Guo. 1995. Evolution and phylogeny of Wolbachia reproductive parasites of arthropods. Proc. Royal Soc. London Series B 261: 55-71. [ Links ]
Williams, J.G.K., A.R. Kubelik, K.J. Livak, J.A. Rafalski & S.V. Tingery. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucl. Acids Res. 18: 6531-6535. [ Links ]
Willams, J.G.K., N.M. Teau, G.J. Puterka & J.R. Nechols. 1992. Use of the random amplified polymorphic DNA polymerase chain reaction (RAPD-PCR) to detect DNA polymporphisms in aphids (Homoptera: Aphididae). Bull. Entomol. Res. 82: 151-159. [ Links ]
Wilson, M. & M. Claridge. 1991. Handbook for the identification of leafhoppers and planthoppers of rice. C.A.B. Wallingford Oxon, International Institute of Entomology (Natural Resources Institute). 142 p. [ Links ]
Zamora, A. 2001. Diversidad morfológica y genética de las especies de Oryza (Poaceae) nativas de Costa Rica. M.Sc. Thesis. Universidad de Costa Rica, Costa Rica. [ Links ]
Zárate, E. 1977. Principales sistemas de vientos que afectan a Costa Rica y sus relaciones con la precipitación. Lic. Tesis en Meteorología. Escuela de Física, Facultad de Ciencias, Universidad de Costa Rica. [ Links ]
Zárate. E. 1978. Comportamiento del viento en Costa Rica. Nota de investigación No.2. Instituto Meteorológico Nacional. San José, Costa Rica. [ Links ]












 uBio
uBio