Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.52 n.3 San José Sep. 2004
Distribution, ecology, life history, genetic variation, and risk of extinction of nonhuman primates from Costa Rica
María E. Zaldívar1 *, Oscar Rocha1,2 , Kenneth E. Glander 3 , Gabriel Aguilar 1 , Ana S. Huertas1 , Ronald Sánchez4 & Grace Wong5
1 Universidad de Costa Rica, Escuela de Biología, Ciudad Universitaria Rodrigo Facio, San José, Costa Rica.
2 Present address Department of Biological Sciences, Kent State University, 256 Cunningham Hall, Kent, OH, 44242. USA.
3 Department of Biological Anthropology and Anatomy, Duke University, Durham, NC, 27708 U.S.A.
4 Departamento de Ciencias Naturales, Sede de Occidente, Universidad de Costa Rica, San Ramón de Alajuela, Costa Rica.
5 Programa de Maestría en Vida Silvestre, Universidad Nacional de Costa Rica, Heredia, Costa Rica.
* Corresponding author: Maria E. Zaldivar. Escuela de Biología, Universidad de Costa Rica, San Pedro, San José, Costa Rica. Tel. (506) 207 4644, Fax (506) 207 4216; marizaldivar@hotmail.com
Received 08-VI-2004. Corrected 27-VIII-2004. Accepted 31-VIII-2004.
Abstract: We examined the association between geographic distribution, ecological traits, life history, genetic diversity, and risk of extinction in nonhuman primate species from Costa Rica. All of the current nonhuman primate species from Costa Rica are included in the study; spider monkeys (Ateles geoffroyi), howling monkeys (Alouatta palliata), capuchins (Cebus capucinus), and squirrel monkeys (Saimiri oerstedii). Geographic distribution was characterized accessing existing databases. Data on ecology and life history traits were obtained through a literature review. Genetic diversity was characterized using isozyme electrophoresis. Risk of extinction was assessed from the literature. We found that species differed in all these traits. Using these data, we conducted a Pearson correlation between risk of extinction and ecological and life history traits, and genetic variation, for widely distributed species. We found a negative association between risk of extinction and population birth and growth rates; indicating that slower reproducing species had a greater risk of extinction. We found a positive association between genetic variation and risk of extinction; i.e., species showing higher genetic variation had a greater risk of extinction. The relevance of these traits for conservation efforts is discussed. Rev. Biol. Trop. 52(3): 679-693. Epub 2004 Dic 15.
Key words: New World monkeys, Alouatta palliata, Ateles geoffroyi, Cebus capucinus, Saimiri oerstedii, isozymes, habitat destruction, biological conservation
Palabras clave: monos del Nuevo Mundo, Alouatta palliata, Ateles geoffroyi, Cebus capucinus, Saimiri oerstedii, isozimas, destrucción del habitat, conservación biológica.
Forest destruction and fragmentation, agricultural practices, hunting, and extraction for pets or research purposes (Mittermeier and Cheney 1987, Rodriguez-Luna et al. 1996) have negatively affected nonhuman primate species in Central America. However, species differ in their responses to these factors. There are four nonhuman primate species in Costa Rica and they all show different risks of extinction. Squirrel monkeys (Saimiri oerstedii) are at greater risk; the IUCN lists them as endangered, with one subspecies (S. o. citrinellus) listed as critically endangered (Mittermeier et al. 1986, Rylands et al. 1995, Rylands 1997, Boinski and Sirot 1997). Squirrel monkeys are the Central American nonhuman primates at greater risk of extinction. Spider monkeys (Ateles geoffroyi) rank second and one subspecies (A. g. panamensis) is considered to be endangered while the other (A. g. ornatus) is described as vulnerable in the IUCN red list (Rylands et al. 1995, Rylands 1997). Howling monkeys (Alouatta palliata) and capuchins (Cebus capucinus) have experienced recent reductions in their population sizes and their geographic distributions but they are not considered vulnerable or endangered by the IUCN specialists (Rylands et al. 1995, Rylands 1997).
Several factors determine the ability of a species to survive environmental disturbance. Rareness is associated with greater risks of extinction (Rabinowitz et al. 1986). Species rareness is determined by the geographic distribution, the diversity of habitats occupied, and population sizes (Rabinowitz et al. 1986, Dobson and Yu 1993). Species with broader geographic distributions, found in more types of habitats, and with larger population sizes, are at lower risks of extinction. Vulnerability is also determined by ecological and life history traits (Johns and Skorupa 1987, Arita et al. 1990, Sorensen and Fedigan 2000). Among nonhuman primates, a larger size and a greater proportion of fruit in the diet are believed to increase the risk of extinction; i.e. large bodied frugivores are more vulnerable than smaller bodied folivores (Johns and Skorupa 1987, Sorensen and Fedigan 2000). Lower fecundity and larger home ranges are also associated with greater risks of extinction (Johns and Skorupa 1987, Mc Farland 1989, Sorensen and Fedigan 2000).
Low levels of genetic diversity are also believed to increase the risk of extinction of threatened or endangered populations (OBrien et al. 1985, Allendorf and Leary 1986, OBrien and Everman 1988, Quattro and Vriejenhoek 1989, OBrien 1994a, 1994b, Lacy 1997). Perhaps the most frequently cited example involves the cheetah; extremely small levels of genetic variation in this species have been associated with low fecundity in captivity and high offspring mortality in the wild (O´Brien et al. 1985, O´Brien and Evermann 1988, O´Brien 1994a, 1994b) (for a criticism of this hypothesis see Caro and Laurenson 1994, Laurenson et al. 1995). Data from several species show that low levels of genetic diversity do not result in low rates of population growth (Bonnel and Selander 1974, Hoelzel et al. 1993, Stewart et al. 1994, see references in Avise 1994). Also, many threatened species retain high levels of genetic diversity (Dinerstein and Mc Cracken 1990, Baker et al. 1993, Hartl and Hell 1994, Tomiuk et al. 1997, Ye et al. 1999, Young et al. 1999). Among primates, microsatellite variation in geographically restricted and endangered bonobos (Pan paniscus) is comparable to that of its widespread congener P. troglodytes (Reinartz et al. 2000). Therefore, there is not a clear correlation between genetic diversity and risk of extinction.
In this study we conducted a literature review to obtain information about the ecology and life history of the nonhuman primate species from Costa Rica. All nonhuman primate species found in Costa Rica are included in the study; i.e., spider monkeys (A. geoffroyi), howling monkeys (A. palliata), capuchins (C. capucinus), and squirrel monkeys (S. oerstedii). We searched existing databases and contacted people working in protected areas to characterize their geographic distribution. In addition, we conducted red cell isozyme electrophoresis to characterize their genetic variation. Using these data, we examined the association between the risk of extinction of nonhuman primates from Costa Rica and their geographic distribution, ecology, life history, and genetic diversity.
Materials and methods
Study species
Geographic distribution: At a regional level, the four species differ in their geographic distribution. Ateles geoffroyi, Alouatta palliata, and Cebus capucinus have a wide geographic distribution (Crockett and Eisenberg 1987, Rylands et al. 1995, Rylands 1997, Crockett 1998, Sorensen and Fedigan 2000). A. geoffroyi are found from Mexico to Panama, A. palliata are found from Mexico to northern Ecuador, and C. capucinus from Honduras to Colombia. Saimiri oerstedii have a very restricted geographic distribution; historically their distribution has been limited to the Pacific wet lowlands of Costa Rica and Panama at altitudes lower than 500 masl, an area smaller than 8000 km2 (Wong 1990a, 1990b, Boinski and Sirot 1997, Crockett 1998, Boinski and Cropp 1999).
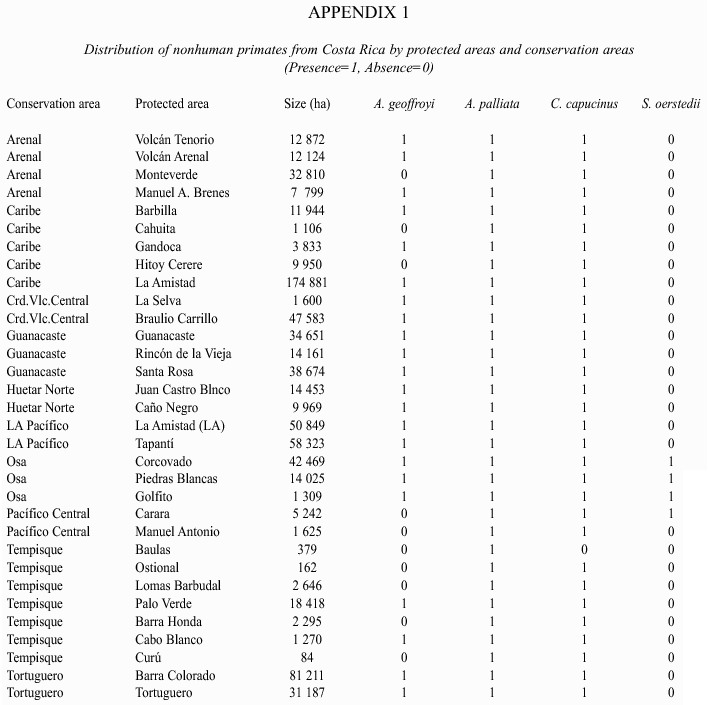
At a local level, the four species also differ in their distribution. Appendix 1 shows the presence or absence of these species within conservation areas and protected areas. Conservation areas are large administrative units that include both protected and non-protected areas. These data underestimate the distribution of these species within the country because only populations from protected areas are included. Nonhuman primates are also found outside protected areas, but it was not possible to verify their presence or absence there.
Overall, A. geoffroyi, C. capucinus, and A. palliata have a wide geographic distribution within the country, whereas S. oerstedii have a very narrow distribution (Wilson 1983, Boza 1992, Rodriguez and Chinchilla 1996, Boinski and Sirot 1997, Carrillo et al. 1999, Saenz et al. 1999, Wong et al. 1999, Mora 2000). A. geoffroyi occur principally in protected areas of larger size and extensive forest coverage. A. geoffroyi are not found in the Central Pacific conservation area, where the protected areas are of smaller size. C. capucinus and A. palliata are found in all conservation areas and most protected areas. S. oerstedii are only found in the Central Pacific and Osa conservation areas located in the central and south Pacific region.
Population sizes: We lack accurate estimates of population sizes for the whole country, but there is published data for populations within some protected areas. A census conducted in 1972 at the Santa Rosa National Park (Freese 1976) reported estimated population sizes of 110 individuals for A. geoffroyi, 85 A. palliata, and 300 C. capucinus. Follow up censuses show that, after almost 30 years of protection within the park and regeneration of the natural habitat, A. palliata and C. capucinus have increased their numbers to 606 and 585 individuals, respectively (Sorensen and Fedigan 2000, Fedigan and Jack 2001). We do not have figures for A. geoffroyi but, according to Sorensen and Fedigan (2000), the population of A. geoffroyi has grown at a slower rate. Censuses conducted in other areas within the Guanacaste region also show that A. palliata are more common, followed by C. capucinus. In all sites censused, A. geoffroyi are the least common. At Palo Verde, Massey (1987) found that the density of A. palliata was 59.3 ind/km2 , for C. capucinus there were about 15.4 ind/km2 , and for A. geoffroyi only 0.62 ind/km2 . At Cabo Blanco, Lippold (1988) found 80 A. palliata, and 54 C. capucinus. Other censuses show fairly large populations of A. palliata. At La Pacífica, the estimated population size has remained around 351 individuals for almost twenty years (Clarke et al. 1986, Clarke and Zucker 1994, Clarke et al. 2002a, 2002b). At La Selva, the population ranged between 105 and 225 individuals (Stoner 1994).
S. oerstedii is believed to have smaller populations than the other species. According to Boinski and Sirot (1997), the current population size of S. o. citrinellus within the protected area of the Manuel Antonio National Park is about 100 individuals, while the population size of S. o. oerstedii within the Corcovado National Park ranges between 200 and 500 individuals.
Habitats occupied: A. geoffroyi, A. palliata, and C. capucinus occupy a variety of altitudinal ranges and habitats, including mangroves, riparian forests, dry and humid forests, low-land, premontane, and montane forests, secondary forests, and forest edges (Eisenberg 1983, Freese 1983, Glander 1983, Sanchez 1991, Stoner 1994, Carrillo et al. 1999, Mora 2000, Clarke et al. 2002a, 2002b). A. geoffroyi are found at altitudes ranging from sea level to 2200 m (Carrillo et al. 1999), but are restricted to large forest fragments and are less common in altered environments (Estrada 1988, Estrada and Coates-Estrada 1996, Carrillo et al. 1999, Sorensen and Fedigan 2000). A. palliata and C. capucinus show comparable altitude ranges (between sea level and 2500 and 3000 m, respectively) (Carrillo et al. 1999), but they can be found in small forest patches and altered environments (Freese 1983, Wilson 1983, Crockett 1998, Horwich 1998, Carrillo et al. 1999). The mean size of protected areas in which A. geoffroyi occurs is 33 529 ha whereas A. palliata, and C. capucinus are found in areas averaging 23 906 and 23 930 ha, respectively (From the values in Appendix 1). S. oerstedii occupy fewer kinds of habitats. They are commonly found at altitudes below 500 m, in secondary forests and forest edges, but they can also be present in habitats showing a mosaic of forest types and in cultivated areas (Boinski 1987, Wong 1990a, 1990b, Carrillo et al. 1999).
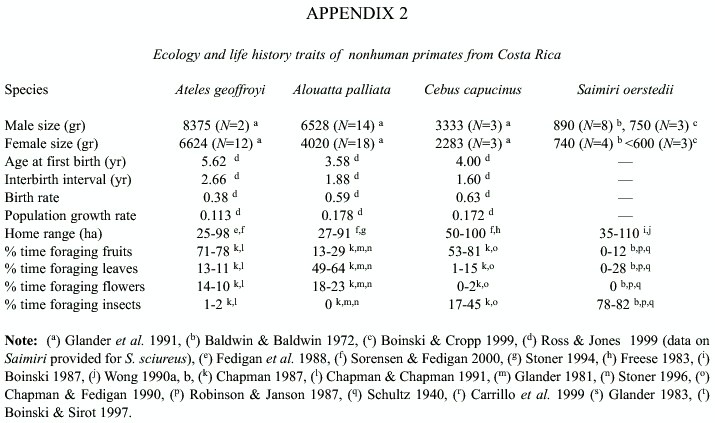
Home range and diet: There is a great degree of variation in home range size within each species (Appendix 2) (Freese 1983, Boinski 1987, Fedigan et al. 1988, Wong 1990a, 1990b, Sanchez 1991, Clarke and Zucker 1994, Stoner 1994, Boinski and Sirot 1997, Sorensen and Fedigan 2000). Overall, home range sizes vary between 25 and 100 ha.
These species also show variation in the proportion of time spent eating fruits, flowers, leaves, or insects (Appendix 2). Within species, the proportion of time eating each kind of fruit item varies spatially and temporally, depending on their availability. A. geoffroyi consume primarily fruits and some leaves and flowers (Chapman 1987, Chapman and Chapman 1991, Strier 1992, Sorensen and Fedigan 2000). A. palliata spend most of their time eating leaves and fruits (Glander 1981, Chapman 1987, Sanchez 1991, Strier 1992, Stoner 1996, Sorensen and Fedigan 2000). C. capucinus eat mostly fruits and insects and sometimes small vertebrates (Chapman 1987, Chapman and Fedigan 1990, Sorensen and Fedigan 2000). S. oerstedii eat mostly insects and some fruits (Schultz 1940, Baldwin and Baldwin 1972, Robinson and Janson 1987, Wong 1990a, 1990b).
Life history traits: The four species differ in their life history traits. Appendix 2 shows that A. geoffroyi and A. palliata are the largest, C. capucinus is intermediate, and S. oerstedii is the smallest (Baldwin and Baldwin 1972, Glander et al. 1991, Boinski and Cropp 1999). It also shows that A. geoffroyi has the lowest reproductive rates (Fedigan and Rose 1995, Ross and Jones 1999, Fedigan and Jack 2001). A. palliata and C. capucinus show higher reproductive rates than A. geoffroyi (Glander 1980, 1992, Clarke and Glander 1984, Crockett and Eisenberg 1987, Robinson and Janson 1987, Fedigan and Rose 1995, Fedigan and Jack 2001). We did not find accurate estimates of reproductive rates for S. oerstedii. Data on S. sciureus, other species in the same genus with similar life history and behavior traits, reveals the highest values for all parameters determining reproductive rates (Boinski and Cropp 1999, Ross and Jones 1999).
Genetic analysis
Sample collection: Samples of S. oerstedii were collected in the Central Pacific Region. All the samples collected belonged to the S. o. citrinellus subspecies. Two samples were collected at the Manuel Antonio National Park and six samples were collected at Jardin Gaia, a wildlife rehabilitation center next to the park, which receives animals confiscated by the Wildlife Service. The Manuel Antonio National Park has the largest extant population of S. o. citrinellus. Samples of A. geoffroyi and C. capucinus were obtained at the Parque Zoológico Nacional Simón Bolívar, San José, during their annual physical examination. All the animals also had been confiscated by the Wildlife Service and came from various regions within the country. The exact location of origin was not known for most of the animals. Animals born in captivity (only one) were not included in this study. We analyzed nine samples of A. geoffroyi and five of C. capucinus.
Field samples were collected using standard dart gun capture techniques (Glander et al. 1991). Samples from captive animals did not require darting. In all cases animals were anesthetized with Telazol (Tilethamine hydrochloride and zolazepam hydrochloride) and blood was drawn from their femoral vein using a Vacutainer with EDTA as an anticoagulant. Darted animals were allowed to recover and then released at the site of capture.
In a previous study we examined blood samples from A. palliata from several sites in Costa Rica (Zaldivar et al. 2003). Atotal of 76 samples were analyzed. Most samples come from the Guanacaste region, located on the Northern Pacific side of the country (61 samples), eight samples were collected in the Atlantic Region and seven samples in the Central Pacific region. Elsewhere we provide the exact location of the sites in which the samples were collected (Zaldivar et al. 2003) . The Atlantic and Pacific Regions are separated by a mountain range running along the center of the country in a North-South direction.
The blood samples were kept cold until processing in the laboratory. Once in the laboratory, they were centrifuged at low speed for about 15 minutes to separate plasma, white cells, and red cells. The red cells were washed twice with isotonic 9% saline solution. Each fraction was stored separately at -20 ºC.
Isozyme analyses: In order to examine the level of genetic variation in each species, we performed electrophoresis of red cell enzymes following the methods described by Harris and Hopkinson (1978), Richardson et al. (1986), and Lima et al. (1990). Electrophoresis was conducted on 9 to 11% starch gels. Electrophoresis was run overnight at constant voltage (60-75 Volts). We examined ten enzyme systems, i.e. CA2, DIA, EST, GPI, LDH, MDH, PGD, PGM, SOD, and TPI. For each red cell enzyme we used the buffers and staining procedures recommended by Harris and Hopkinson (1978). We recorded the genotype of each individual.
Genetic analyses were conducted using the program POPGENE 1.32 (Yeh et al. 1999). We determined the levels of genetic diversity for each species using common indicators such as observed number of alleles, effective number of alleles, percentage polymorphic loci, observed heterozygosity, and expected heterozygosity.
Association between risk of extinction and ecological, life history, and genetic traits: The four primate species included in this study differ in their conservation status. S. oerstedii is considered to be endangered. This species has a very narrow geographic distribution, natural habitats have been destroyed through deforestation, and in some areas population sizes are small. All other species have wide geographic distributions within the country and the region, yet the conservation status of A. geoffroyi is considered to be vulnerable.
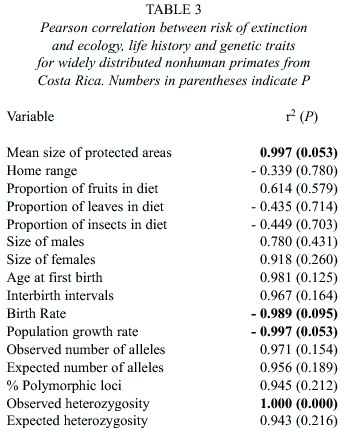
In order to determine which factors may explain the higher risk of extinction of A. geoffroyi compared to A. palliata and C. capucinus, we conducted a Pearson correlation analysis and examined the association between risk of extinction and ecological traits (mean size of protected areas in which the species is present, size of home range, proportion of fruits in diet, proportion of leaves, proportion of insects), life history (size of males, size of females, age at first birth, interval between births, birth rate, intrinsic rate of population growth), and genetic diversity (observed number of alleles, effective number of alleles, percentage polymorphic loci, observed heterozygosity, and expected heterozygosity). The risk of extinction was assigned a categorical value based on the conservation status determined by Rylands et al. (1995) and Rylands (1997). C. capucinus and A. palliata have the lowest risk of extinction and were assigned a value of 1, A. geoffroyi has a higher risk was assigned a value of 2.
Results
Isozyme analyses
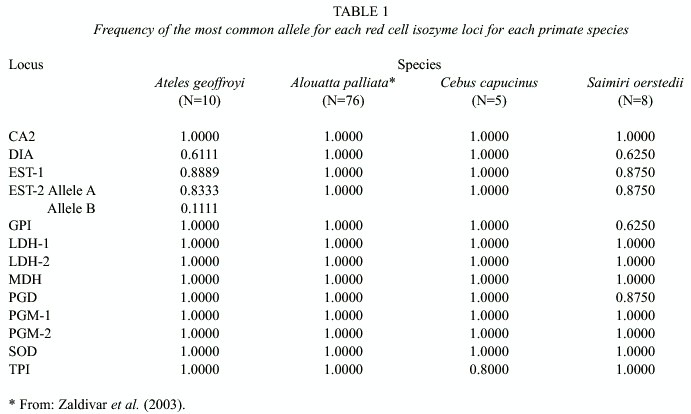
A total of 13 putative loci were examined. Seven loci were monomorphic (CA2, LDH-1, LDH-2, MDH, PGM-1, PGM-2, and TPI); i.e. they showed only one electrophoretic variant. Six loci showed two or more variants (DIA, EST-1, EST-2, GPI, PGD, and SOD). Table 1 shows the frequency of the most common allele for all loci. S. oerstedii showed the greatest number of variable loci; 5 of the 13 loci studied had more than one variant (DIA, EST-1, EST-2, GPI, and PGD). A. geoffroyi had three loci showing more than one electrophoretic variant (DIA, EST-1, and EST-2). C. capucinus showed only one loci with more than one electrophoretic variant (SOD), but the sample size was very small (only five individuals). A. palliata, despite their much larger sample size, showed no variation and this was true for all the enzymes analyzed; all the individuals examined showed the same electrophoretic pattern at all loci.
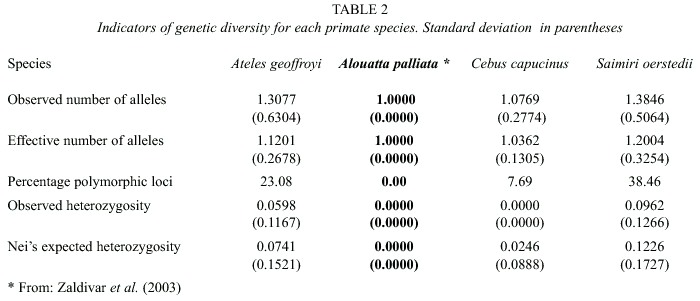
The four species studied have different patterns of genetic variation according to the values of the indicators of genetic diversity shown in Table 1, i.e. observed number of alleles, effective number of alleles, percentage polymorphic loci, observed heterozygosity, and expected heterozygosity. For all indicators of genetic diversity, S. oerstedii show the greatest values, A. geoffroyi rank second, C. capucinus rank third, and A. palliata rank last, since they show no genetic variation for any of the loci examined (Table 2).
Association between risk of extinction and ecological, life history, and genetic traits: Among widely distributed species, we found a negative association between risk of extinction and mean size of protected areas in which the species are found (Table 3); where species present in areas of larger mean size are at greater risk of extinction and species found in areas of smaller sizes are at lower risk. For all other ecological variables we did not find a statistically significant association with risk of extinction (Table 3). For the correlation analyses between risk of extinction and proportion of fruits in diet, proportion of leaves, proportion of insects, and home range, we used the midvalue of the ranges described in the Appendix. We did not find a statistically significant association between risk of extinction and size of males, size of females, age at first birth, and interval between births (Table 3). But we found that risk of extinction is negatively associated with birth rate and rate of population growth (table 3); where species with greater birth and population growth rates are at lower risk of extinction and species with smaller birth and population growth rates are at greater risk. For most indicators of genetic diversity we found no statistically significant association between risk of extinction and genetic diversity, but species with greater observed heterozygosity were at greater risk than less diverse species.
Discussion
Our analysis of isozyme variation revealed that S. o. citrinellus and A. geoffroyi showed high levels of isozyme variation whereas A. palliata and C. capucinus showed very low levels of genetic variation. A. palliata did not show variation for any of the isozymes studied, even though we examined more samples of this species than for any other (Zaldivar et al. 2003).
The values of isozyme variation that we find for S. o. citrinellus, A. geoffroyi, and C. capucinus concur with the results reported for these same groups in South America. South American Saimiri showed genetic variation for EST, GPI, LDH, MDH, PGD, and PGM (Vandeberg et al. 1990, Silva et al. 1993). Heterozygosity estimates ranged between 0.086 and 0.025 (Vandeberg et al. 1990, Silva et al. 1993) and are comparable to the value reported by us. South American spider monkeys (A. paniscus) showed variation in one out of 20 loci (CA2) and A. chamek showed variation in three (ADA, ESD, and GLO) (Sampaio et al. 1993). Average heterozygosity was slightly lower than the value found by us (0.06) and it ranged between 0.021 and 0.031 (Sampaio et al. 1993). South American capuchins showed low levels of genetic variation. Sampaio et al. (1993) found that C. apella paraguayanus was polymorphic for two loci (GLO and GPI) and showed an average heterozygosity of 0.014.
The absence of genetic variation that we found for A. palliata (Zaldivar et al. 2003) is comparable to the low values of genetic diversity previously reported for this and other Central American populations, and contrasts with the high values reported for South American Alouatta. Genetic studies of A. palliata in Central America indicate that this species has extremely low levels of isozyme variation. Malmgren and Brush (1978), using two-dimensional electrophoresis, found 2 of 15 polymorphic loci, including MDH, reported as monomorphic by us. Froelich and Thorington (1982) reported 3 of 25 polymorphic loci (ADA and two serum proteins). Ellsworth (2000) analyzed microsatellite variation in A. palliata from Panama, Costa Rica, and Mexico, and found that this species has extremely low genetic variability; the mean number of alleles was 6.3 and the mean observed heterozygosity was 0.35 in a sample of 117 individuals. Genetic studies of the other Central American howler, A. pigra, also found low levels of isozyme and DNA variation (James et al. 1997, Ellsworth 2000). A. pigra shows variation in 2 of 36 loci, including GPI, monomorphic in A. palliata (James et al. 1997). It also shows low levels of microsatellite variation (Ellsworth 2000); in a sample of 28 individuals, the mean number of alleles was 3.8 and the mean observed heterozygosity was 0.45. Genetic studies of South American Alouatta indicate that these species have very high levels of genetic variation. In A. seniculus, Pope (1992) found variation in 10 out of 29 loci and Lima et al. (1990) found variation in 9 out of 26 loci. A. belzebul showed comparably high levels of genetic variation (Sampaio 1996). Ellsworth (2000), using microsatellite analysis, found that the mean number of alleles per individual in A. seniculus (0.71) is much higher than in A. palliata (0.165). Mean observed heterozygosity values were also higher; 0.56 in a sample of six individuals (Ellsworth 2000).
Among the three species with a wider geographic distribution, we found no association between risk of extinction and most ecological variables such as degree of frugivory or home range size. Home range and diet were highly variable, both between species and within species, and they show both temporal and spatial variation (Schultz 1940, Baldwin and Baldwin 1972, Glander 1981, Freese 1983, Boinski 1987, Chapman 1987, Robinson and Janson 1987, Fedigan et al. 1988, Chapman and Fedigan 1990, Wong 1990a, 1990b, Chapman and Chapman 1991, Sanchez 1991, Strier 1992, Clarke and Zucker 1994, Stoner 1994, 1996, Boinski and Sirot 1997, Sorensen and Fedigan 2000). Variation within species would obscure any possible association due to variation between species. We do find an association between risk of extinction and mean size of protected areas in which the species is present; A. geoffroyi are present in areas of larger average mean size and are at greater risk of extinction. A. geoffroyi are seldom present in protected areas of smaller size and they seem to require larger areas of forest.
Also, among the three species with a wider geographic distribution, we found no association between risk of extinction and most life history traits. But we did observe that lower birth rates and lower population growth rates were associated with greater risks of extinction. These results allow us to explain the vulnerability of A. geoffroyi. Our interpretation agrees with those of other authors who find that lower rates of population growth, limit the potential of A. geoffroyi populations to recover rapidly after experiencing reductions in population size (Mc Farland 1989, Sorensen and Fedigan 2000).
Finally, we found no association between risk of extinction and genetic diversity. Contrary to the prediction that low levels of genetic diversity increase extinction risks, we found that less variable species, such as C. capucinus and A. palliata, had lower risks of extinction than more variable species, such as S. oerstedii and A. geoffroyi. Despite their low levels of genetic variation C. capucinus and A. palliata are very resilient and recover quickly from environmental disturbance (Pope 1995, Crockett 1998, Horwich 1998, Sorensen and Fedigan 2000). Lack of genetic variation has not limited the capacity of A. palliata and C. capucinus to occupy a variety of habitats, including altered environments and small forest patches. Also, it has not limited their ability to recover from habitat destruction (Pope 1995, Fedigan et al. 1998, Sorensen and Fedigan 2000). A. palliata from the population at La Pacífica, Costa Rica, have experienced rapid recovery from habitat deforestation and their population size has remained stable (Clarke et al. 2002a, 2002b). A. palliata from the Santa Rosa National Park show a seven-fold increase in size in 28 years since the establishment of protection and regeneration of the dry forest (Fedigan et al. 1998, Fedigan and Jack 2001). C. capucinus doubled their population size in this same period (Sorensen and Fedigan 2000).
To conclude, geographic distribution and life history traits seem to be more important determinants of extinction risks than genetic variation, at least for nonhuman primates from Costa Rica. Conservation efforts should emphasize the relevance of these variables. Our data support the need to expand and enforce protection measures for squirrel monkeys. Squirrel monkeys are the most endangered primate species in Central America (Rylands et al. 1995, Rylands 1997). Their narrow geographic distribution and habitat range, coupled to extensive habitat destruction and fragmentation in the region, are responsible for the low numbers of populations and individuals remaining (Boinski and Sirot 1997). Spider monkeys are also very vulnerable to disturbances due to their low rate of population growth. The successful recovery of primate populations in the Santa Rosa National Park (Fedigan et al. 1998, Sorensen and Fedigan 2000, Fedigan and Jack 2001) should encourage further habitat protection and restoration efforts.
Acknowledgments
The authors thank Philip Hedrick, Jorge Azofeifa, Mayrelith Artavia, and Ana Araya, for providing useful comments on a previous version of this manuscript. Jose Manuel Mora and Eduardo Carrillo for providing help with the geographic distribution and useful comments. Luis Castro for assistance in the lab, Gustavo Gutiérrez, Isabel Salas and many student volunteers for assistance in the field. Special thanks to the owner and manager of Hacienda La Pacifica for allowing us to work on the ranch. Yolanda Matamoros and Danilo Leandro at the Parque Zoológico Nacional Simón Bolívar, and Dario Castelfranco at Jardin Gaia, for providing us blood samples from their captive animals. This work was funded in part by NSF Grants DBC-9118876
and SBR-9601766 to Mark Teaford and KEG, Universidad de Costa Rica Dean of Research Grant 111-93-316 to MEZ, and Third World Academy of Sciences Grant 95-364 RG/BIO/LA to MEZ. This research benefited greatly from a visit to Duke University by MEZ funded by the M. I. R. T. Program at this institution. The authors also thank the Office of International Affairs of the Universidad de Costa Rica for a fellowship to MEZ and OJR, which was instrumental for the preparation of this manuscript.
Resumen
Se estudió la asociación entre la distribución geográfica, algunos rasgos ecológicos, las historias de vida, la diversidad genética y el riesgo de extinción, en primates no humanos de Costa Rica. Se incluyen todas las especies de primates no humanos del país: los monos araña (Ateles geoffroyi), congo (Alouatta palliata), cara blanca (Cebus capucinus), y tití (Saimiri oerstedii). La distribución geográfica se caracterizó utilizando principalmente bases de datos existentes. Se obtuvo información acerca de sus características ecológicas y de historias de vida mediante una revisión bibliográfica. Se estudió su diversidad genética utilizando electroforesis de isoenzimas. El riesgo de extinción se evaluó usando información bibliográfica. Se encontró que las cuatro especies presentaban variación en todos estos rasgos. Con estos datos, se realizó una correlación de Pearson entre el riesgo de extinción y las variables indicadoras de la distribución geográfica, los rasgos ecológicos, las historias de vida y la diversidad genética, para aquellas especies con una amplia distribución geográfica. Se encontró una asociación entre el riesgo de extinción y la natalidad y la tasa de crecimiento poblacional; las especies con menor natalidad y menor tasa de crecimiento poblacional tenían mayor riesgo de extinción. Se encontró una asociación positiva entre la diversidad genética y el riesgo de extinción; las especies con mayor diversidad genética tenían mayor riesgo de extinción. Se discute la importancia de estos rasgos para la conservación de estas especies.
References
Allendorf, F.W. & R.F. Leary. 1986. Heterozygosity and fitness in natural populations of animals, pp. 57-76. In M. Soule (ed.). Conservation biology: the science of scarcity and diversity. Sinauer, Massachusetts. [ Links ]
Arita, H., J.G. Robinson & K.H. Redford. 1990. Rarity in neotropical forest mammals and its ecological correlates. Conserv. Biol. 4: 181-192. [ Links ]
Avise, J.C. 1994. Molecular markers, natural history, and evolution. Chapman and Hall, New York. 511 p. [ Links ]
Baker, C.S., A. Perry, J.L. Bannister & M.T. Weinrich, R.B. Abernethy. 1993. Abundant mitochondrial DNA variation and worldwide population structure in humpback whales. Proc. Natl. Acad. Sci. USA 90: 8238-8243. [ Links ]
Baldwin J.D. & J. Baldwin. 1972. The ecology and behavior of squirrel monkeys (Saimiri oerstedii) in a natural forest in western Panama. Folia Primatol. 18: 161-184. [ Links ]
Boinski, S. 1987. Habitat use by squirrel monkeys (Saimiri oerstedi) in Costa Rica. Folia Primatol. 49: 151-167. [ Links ]
Boinski, S. & L. Sirot. 1997. Uncertain conservation status of Squirrel monkeys in Costa Rica, Saimiri oerstedi oerstedi and Saimiri oerstedi citrinellus. Folia Primatol. 68: 181-193. [ Links ]
Boinski, S. & S.J. Cropp. 1999. Disparate data sets resolve squirrel monkey (Saimiri) taxonomy: implications for behavioral ecology and medical usage. Int. J. Primatol. 20: 237-256. [ Links ]
Bonnel, M.L. & R.K. Selander. 1974. Elephant seals: genetic variation and near extinction. Science 184: 908-909. [ Links ]
Boza, M.A. 1992. Parques Nacionales de Costa Rica. Incafo, Santo Domingo, Heredia, Costa Rica. 91 p. [ Links ]
Caro, T.M. & M.K. Laurenson. 1994. Ecological and genetic factors in conservation: a cautionary tale. Science 263: 485-48. [ Links ]
Carrillo, E., G. Wong & J.C. Saenz. 1999. Mamíferos de Costa Rica. Inbio, Santo Domingo, Heredia, Costa Rica. 248 p. [ Links ]
Chapman, C.A. 1987. Flexibility in diets of three species of Costa Rican primates. Folia Primatol. 49: 90-105. [ Links ]
Chapman, C.A. & L.M. Fedigan. 1990. Dietary differences between neighboring Cebus capucinus groups: local traditions, food availability or responses to food profitability? Folia Primatol. 54: 177-186. [ Links ]
Chapman, C.A. & L.J. Chapman. 1991. The foraging itinerary of spider monkeys: when to eat leaves? Folia Primatol. 56: 162-166. [ Links ]
Clarke, M.R. & K.E. Glander. 1984. Female reproductive success in a group of free-ranging howling monkeys (Alouatta palliata) in Costa Rica, pp. 111-126. In M.F. Small (ed.). Female primates: studies by women primatologists. Alan R. Liss Inc, New York. [ Links ]
Clarke, M.R., E.L. Zucker & N.J. Scott. 1986. Population trends of the mantled howler groups at La Pacifica, Guanacaste, Costa Rica. Am. J. Primatol. 11: 79-88. [ Links ]
Clarke, M.R. & E.L. Zucker. 1994. Survey of the howling monkey population at La Pacifica: a seven year follow- up. Int. J. Primatol. 15: 61-73. [ Links ]
Clarke, M.R., D.A. Collins & E.L. Zucker. 2002a. Responses to deforestation in a group of mantled howlers (Alouatta palliata) in Costa Rica. Int. J. Primatol. 23: 365-381. [ Links ]
Clarke, M.R., C.M. Crockett, E.L. Zucker & M. Zaldivar. 2002b. Mantled howler population of Hacienda La Pacifica, Costa Rica, between 1991 and 1998: effects of deforestation. Am. J. Primatol. 56: 155-163. [ Links ]
Crockett, C.M. 1998. Conservation biology of the genus Alouatta. Int. J. Primatol. 19: 549-577. [ Links ]
Crockett, C.M. & J.F. Eisenberg. 1987. Howlers: variation in group size and demography, pp. 54-68. In B.B. Smuts, D.L. Cheney, R.M. Seyfarth, R.W. Wrangham & T.T. Struhsaker (eds.). Primate Societies. University of Chicago, Chicago. [ Links ]
Dinerstein, E. & G.F. McCracken. 1990. Endangered greater one-horned rhinoceros carry high levels of genetic variation. Conserv. Biol. 4: 417-422. [ Links ]
Dobson, F.S. & J. Yu. 1993. Rarity in neotropical forest mammals revisited. Conserv. Biol. 7: 586-591. [ Links ]
Eisenberg, J.F. 1983. Ateles geoffroyi (mono araña, mono colorado, spider monkey). In D.H. Janzen (ed.). Costa Rican natural history, pp. 451-453. University of Chicago, Chicago. [ Links ]
Ellsworth, J.A. 2000. Molecular evolution, social structure, and phylogeography of the mantled howler monkey (Alouatta palliata). Ph D Thesis, University of Nevada, Reno, Nevada. 179 p. [ Links ]
Estrada, A. 1988. Tropical rainforest conversion and perspectives in the conservation of wild primates (Alouatta and Ateles) in Mexico. Am. J. Primatol. 14: 315-317. [ Links ]
Estrada, A. & R.M. Coates-Estrada. 1996. Tropical rain forest fragmentation and wild populations of primates at Los Tuxlas. Int. J. Primatol. 5: 759-783. [ Links ]
Fedigan, L.M., L. Fedigan, C.A. Chapman & K.E. Glander. 1988. Spider monkey home ranges: a comparison of radio telemetry and direct observation. Am. J. Primatol. 16: 19-29. [ Links ]
Fedigan, L.M. & L.M. Rose. 1995. Interbirth interval variation in three sympatric species or neotropical monkeys. Am. J. Primatol. 37: 9-24. [ Links ]
Fedigan, L.M., L.M. Rose & R.A. Morera. 1998. Growth of mantled howler groups in a regenerating Costa Rican dry forest. Int. J. Primatol. 19: 405-432. [ Links ]
Fedigan, L.M. & K. Jack. 2001. Neotropical primates in a regenerating Costa Rican dry forest: a comparison of howler and capuchin population patterns. Int. J. Primatol. 22: 689-713. [ Links ]
Freese, C. 1976. Censusing Alouatta palliata, Ateles geoffroyi, and Cebus capucinus in the Costa Rican dry forest, pp. 4-9. In R.W. Thorington & P.G. Heltne (eds.). Distribution and abundance of Neotropical primates. National Academy of Sciences, Washington, D. C. [ Links ]
Freese, C. 1983. Cebus capucinus (mono cara blanca, white-faced capuchin), pp. 458-460. In D.H. Janzen (ed.). Costa Rican natural history. University of Chicago, Chicago. [ Links ]
Froelich, J.W. & R.W. Thorington. 1982. The genetic structure and socioecology of howler monkeys (Alouatta palliata) on Barro Colorado Island, pp. 291-305. In E.G. Leigh, A.S. Rand & D.M. Windsor (eds.). The Ecology of a Tropical Forest. Seasonal rhythms and long-term changes. Smithsonian Institution, Washington D.C. [ Links ]
Glander, K.E. 1980. Reproduction and population growth in free-ranging mantled howling monkeys. Am. J. Phys. Anthropol. 53: 25-36. [ Links ]
Glander, K.E. 1981. Feeding patterns in mantled howling monkeys, pp. 231-259. In A. Kamil & T.D. Sargent (eds.). Foraging behavior: ecological, ethological, and psychological approaches. Garland, New York. [ Links ]
Glander, K.E. 1983. Alouatta palliata (Congo, howling monkey, howler monkey) , pp.448-449. In D.H. Janzen (ed.). Costa Rican natural history. University of Chicago, Chicago. [ Links ]
Glander, K.E. 1992. Dispersal patterns in Costa Rican howling monkeys. Int. J. Primatol. 13: 415-436. [ Links ]
Glander, K.E., L.M. Fedigan, L. Fedigan & C. Chapman. 1991. Field methods for capture and measurement of three monkey species in Costa Rica. Folia Primatol. 57: 70-82. [ Links ]
Harris, H. & D.A. Hopkinson. 1978. Handbook of Enzyme Electrophoresis in Human Genetics. North Holland, Oxford. [ Links ]
Hartl, G.B. & P. Hell. 1994. Maintenance of high levels of allelic variation in spite of a severe population bottleneck in population size: the brown bear (Ursus arctos) in the Western Carpathians. Biodivers. Conserv. 3: 546-554. [ Links ]
Hoelzel, A.R., J. Halley, C. Campagna, T. Arnborn & B. Le Boeuf. 1993. Elephant seal genetic variation and the use of simulation models to investigate historical population bottlenecks. J. Hered. 84: 443-449. [ Links ]
Horwich, R.H. 1998. Effective solutions for howler conservation. Int. J. Primatol. 19: 579-598. [ Links ]
James, R.A., P.L. Leberg, J.M. Quattro & R. Vrijenhoek. 1997. Genetic diversity in Black Howler monkeys (Alouatta pigra) from Belize. Am. J. Phys. Anthropol. 102: 329-336. [ Links ]
Johns, A.D. & J.P. Skorupa. 1987. Responses of rain-forest primates to habitat disturbance: a review. Int. J. Primatol. 8: 157-191. [ Links ]
Lacy, R.C. 1997. Importance of genetic variation to the viability of mammalian populations. J. Mammal. 78: 320-355. [ Links ]
Laurenson, M.K., N. Wielebnowsky & T.M. Caro. 1995. Extrinsic factors and juvenile mortality in cheetahs. Conserv. Biol. 9: 1329-1331. [ Links ]
Lima, M.M.C., M.I.C. Sampaio, M.P.C. Schneider, W. Scheffrahn, H. Schneider & F.M. Salzano. 1990. Chromosome and protein variation in red howler monkeys. Rev. Bras. Genet. 13: 789-802. [ Links ]
Lippold, L.K. 1988. A census of primates in Cabo Blanco Absolute Reserve, Costa Rica. Brenesia 29: 101-105. [ Links ]
Malmgren, L.A. & A.H. Brush. 1978. Isozymes and plasma proteins in eight troops of golden mantled howling monkeys (Alouatta palliata), pp. 283-285. In D.J. Chivers and R.A. Joysey (eds.). Recent advances in primatology III, Evolution. Academic, London. [ Links ]
Massey, A. 1987. A population survey of Alouatta palliata, Cebus capucinus, and Ateles geoffroyi at Palo Verde, Costa Rica. Rev. Biol. Trop. 35: 345-347. [ Links ]
Mc Farland, M. 1989. Environmental determinants of population densities in Ateles. Primate Conserv. 9: 74-79. [ Links ]
Mittermeier, R.A., J.F. Oates, A.E. Eudey & J. Thornback. 1986. Primate conservation, pp. 3-72. In G. Mitchell & J. Erwin (eds.). Comparative Primate Biology. Vol. 2. Behavior, Conservation, and Ecology. A. R. Liss, New York. [ Links ]
Mittermeier, R.A. & D.L. Cheney. 1987. Conservation of primates and their habitats, pp. 477-490. In B.B. Smuts, D.L. Cheney, R.M. Seyfarth, R.W. Wrangham & T.T. Struhsaker (eds.). Primate Societies. University of Chicago, Chicago. [ Links ]
Mora, J.M. 2000. Mamíferos silvestres de Costa Rica. UNED, San Jose, Costa Rica. 220 p. [ Links ]
OBrien, S.J. 1994a. A role for molecular genetics in biological conservation. Proc. Natl. Acad. Sci. USA 91: 5748-5755. [ Links ]
OBrien, S.J. 1994b. Genetic and phylogenetic analyses of endangered species. Ann. Rev. Genet. 28: 467-489. [ Links ]
OBrien, S.J., M.E. Roelke, L. Marker, A. Newman, C.A. Winkler, D. Meltzer, L. Colly, J.F. Evermann, M. Bush & D.E. Wildt. 1985. Genetic basis for species vulnerability in the Cheetah. Science 227: 1428- 1434. [ Links ]
OBrien, S.J. & J.F. Everman. 1988. Interactive influence of infectious disease and genetic diversity in natural populations. Trends Ecol. Evol. 3: 254-259. [ Links ]
Pope, T.R. 1992. The influence of dispersal patterns and mating system on genetic differentiation within and between populations of the red howler monkey (Alouatta seniculus). Evolution 46: 1112-1128. [ Links ]
Pope, T.R. 1995. Socioecology, population fragmentation, and patterns of genetic loss in endangered primates, pp. 119-159. In J.C. Avise & J.L. Hamrick (eds.). Conservation genetics: case histories from nature. Chapman and Hall, New York. [ Links ]
Quattro, J.M. & R.C. Vriejenhoek. 1989. Fitness differences among remnant populations of the endangered Sonoran topminow. Science 245: 976-978. [ Links ]
Rabinowitz, D., S. Cairns & T. Dillon. 1986. Seven forms of rarity and their frequency in the flora of the British Isles. In M. Soule (ed.). Conservation biology: the science of scarcity and diversity, pp. 184-204. Sinauer, Sunderland, Massachusetts. [ Links ]
Reinartz, G.E., J.D. Karron, R.B. Phillips & J.L. Weber. 2000. Patterns of microsatellite polymorphism in the range-restricted bonobo (Pan paniscus): considerations for interspecific comparisons with chimpanzees (P. troglodytes). Mol. Ecol. 9: 315-328. [ Links ]
Richardson, B.J., P.R. Baverstock & M. Adams. 1986. Allozyme electrophoresis. A handbook for animal systematics and population studies. Academic, San Diego. 410 p. [ Links ]
Robinson, J.G. & C.H. Janson. 1987. Capuchins, squirrel monkeys, and atelines: socioecological convergence with Old World primates, pp. 69-82. In B.B. Smuts, D.L. Cheney, R.M. Seyfarth, R.W. Wrangham & T.T. Struhsaker (eds.). Primate Societies. University of Chicago, Chicago. [ Links ]
Rodriguez-Luna, E., L. Cortes-Ortiz, R. Mittermeier & A. Rylands. 1996. Plan de acción para los primates mesoamericanos. IUCN/SSC Primates Specialist Group, Xalapa, Veracruz, Mexico. [ Links ]
Rodríguez, J. & F. Chinchilla. 1996. Lista de mamíferos de Costa Rica. Rev. Biol. Trop. 44: 877-890. [ Links ]
Ross, C. & K.E. Jones. 1999. Socioecology and the evolution of primate reproductive rates, pp. 73-110. In P.C. Lee (ed.). Comparative primate socioecology. Cambridge University, Massachusetts. [ Links ]
Rylands, A.B., R.A. Mittermeier & E. Rodriguez-Luna. 1995. Aspecies list for the New World primates (Platyrrhini): distribution by country, endemism, and conservation status, according to the Mace-Lande system. Neotrop. Prim. Supp. 3: 113-163. [ Links ]
Rylands, A.B. 1997. Conservation of neotropical primates: threatened species and an analysis of primate diversity by country and region. Folia Primatol. 68: 134-160. [ Links ]
Saenz, J.C., E. Carrillo & G. Wong. 1999. Mamíferos del area de conservación Arenal. Inbio, Santo Domingo, Heredia. 130 p. [ Links ]
Sampaio, M.I.C. 1996. Taxonomy of the Alouatta seniculus group: biochemical and chromosome data. Primates 37: 65-73. [ Links ]
Sampaio, M.I.C., M.P.C. Schneider & H. Schneider. 1993. Contribution of genetic distances studies to the taxonomy of Ateles, particularly Ateles paniscus paniscus and Ateles paniscus chamek. Am. J. Primatol. 14: 895-903. [ Links ]
Sánchez, R. 1991. Comportamiento y dieta del mono congo (A. palliata) en el bosque premontano de San Ramón, Costa Rica. Tesis de Maestría, Universidad Nacional, Heredia, Costa Rica. 108 p. [ Links ]
Schultz, A.H. 1940. The size of the orbit and of the eye in primates. Am. J. Phys. Anthropol. 26: 389-408. [ Links ]
Silva, B.T.F., M.I.C. Sampaio, H. Schneider, M.P.C. Schneider, E. Montoya, F. Encarnacion, S.M. Callegari-Jacques & F. Salzano. 1993. Protein electrophoretic variability in Saimiri and the question of its species status. Am. J. Primatol. 29: 183-193. [ Links ]
Sorensen, T.C. & L.M. Fedigan. 2000. Distribution of three monkey species along a gradient of regenerating tropical dry forest. Biol. Conserv. 92: 227-240. [ Links ]
Stewart, B.S., P.K. Yochem, H.R. Huber, R.L. De Long, R.J. Jameson, W.J. Sydeman, S.G. Allen & B.J. Le Boeuf. 1994, pp. 29-48. In B.J. Le Boeuf & R.M. Laws (eds.). Elephant seals: population, ecology, behavior, and physiology. University of California, Berkeley. [ Links ]
Stoner, K.E. 1994. Population density of the mantled howler monkey (Alouatta palliata) at La Selva Biological Reserve, Costa Rica: a new technique to analyze census data. Biotropica 26: 332-340. [ Links ]
Stoner, K.E. 1996. Habitat selection and seasonal patterns of activity and foraging of mantled howling monkeys (Alouatta palliata) in Northeastern Costa Rica. Int. J. Primatol. 17: 1-30. [ Links ]
Strier, K.B. 1992. Atelinae adaptations: behavioral strategies and ecological constraints. Am. J. Phys. Anthropol. 88: 515-524. [ Links ]
Tomiuk, J., L. Bachmann, M. Leipoldt, J.U. Ganzhorn, R. Ries, M. Weis & V. Loeschcke. 1997. Genetic diversity of Lepilemur mustelinus ruficaudatus, a nocturnal lemur of Madagascar. Conserv. Biol. 11: 491-497. [ Links ]
Vandeberg, J.L., S. Williams-Blangero, C.M. Moore, M.L. Cheng & C.R. Abee. 1990. Genetic relationships among the three squirrel monkey types: implications for taxonomy, biomedical research, and captive breeding. Am. J. Primatol. 22: 101-111. [ Links ]
Wilson, D.E. 1983. Checklist of mammals, pp. 443-447. In D.H. Janzen (ed.). Costa Rican natural history. University of Chicago, Chicago. [ Links ]
Wong, G. 1990a. Ecología del mono tití (Saimiri oerstedi citrinellus) en el Parque Nacional Manuel Antonio, Costa Rica. Tesis de Licenciatura, Universidad Nacional, Heredia, Costa Rica. 57 p. [ Links ]
Wong, G. 1990b. Uso del habitat, estimación de la composición y densidad poblacional del mono tití (Saimiri oerstedi citrinellus) en la zona de Manuel Antonio, Quepos, Costa Rica. Tesis de Maestría, Universidad Nacional, Heredia, Costa Rica. 78 p. [ Links ]
Wong, G., J.C. Saenz & E. Carrillo. 1999. Mamíferos del parque Nacional Corcovado. Inbio, Santo Domingo, Heredia. 118 p. [ Links ]
Ye, S., K. Wang, D. Hong, W. Zhang & Y. Zu. 1999. Comparisons of genetic diversity in the endangered Adenophora lobophylla and its widespread congener, A. potaninii. Conserv. Biol. 13: 509-513. [ Links ]
Yeh, F.C., R. Yang & T. Boyle. 1999. Popgene version 1.31: Microsoft windows-based freeware for population genetic analysis. University of Alberta, Edmonton, Canada. [ Links ]
Young, A.G., A.H.D. Brown & F.A. Zich. 1999. Genetic structure of fragmented populations of the endangered daisy Rutidosis leptorrhynchoides. Conserv. Biol. 13: 256-265. [ Links ]
Zaldivar, M.E., K.E. Glander, O.J. Rocha, G. Aguilar, E. Vargas, G.A. Gutierrez-Espeleta & R. Sanchez. 2003. Genetic variation of mantled howler monkeys (Alouatta palliata) from Costa Rica. Biotropica 35: 375-381. [ Links ]