Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.52 n.1 San José Mar. 2004
(Gastropoda: Muricidae)
Ludwig C. A. Naegel
Centro Interdisciplinario de Ciencias Marinas. Instituto Politécnico Nacional (CICIMAR/IPN) LA PAZ, B.C.S. 23096 México; lnaegel@cibnor.mx
Abstract
The spawning of the muricid gastropod Plicopurpura pansa in the laboratory at 22-23°C is described. Females deposited 1-20 capsules daily for at least 20 weeks, and produced up to 150 capsules each per spawning season. During spawning, egg clusters were formed consisting of hundreds of capsules of different ages deposited by different females. Each egg capsule contained an average of 436 embryos (± s.d. 213.6, range: 95- 1 092, n = 50). Embryos developed without nurse eggs. After six to eight weeks of intracapsular, lecithotrophic development, planktotrophic veligers hatched with two fully developed velar lobes.
Key words: Spawning, Purple snail, Plicopurpura pansa, Muricidae, Gastropoda.
The purple snail, Plicopurpura pansa (Gould, 1853), is a predator that inhabits the high intertidal of rocky shores exposed to the open sea with high impact waves. The range of P. pansa extends in the Pacific from the north-west coast of Mexico (Baja California Sur) (Clench 1947, Keen 1971) to northern Peru (Peña 1970, Paredes et al. 1999).
Since pre-Columbian times, "Tyrian purple" of P. pansa has been exploited by indigenous communities on the Pacific coast for dying traditional dresses (Martens, v. 1874, 1898, Nutall 1909, Jackson 1917, Born 1936, Gerhard 1962, Turok et al. 1988). However, the tradition of dying material with the colorant of the purple snail has survived only in remote Pacific regions of Mexico (in the States of Oaxaca and Michoacan) and with the indigenous community of the Borucas in Costa Rica (Turok 1999). These practices represent the survival of knowledge of considerable antiquity and it is feared that this old tradition will be lost in the near future (Thompson 1994). In recent years the commercial exploitation of the purple snails for "Tyrian purple" to dye kimonos had reached such levels as to threaten the survival of the species. In 1988 P. pansa was declared a protected species by the Mexican government (Anonymous 1988).
Despite the tradition of exploiting P. pansa for its chromogens, and recent concerns about the future survival of this species, little is known about the principal life-history features, like reproduction, larval development, requirements of larvae and juveniles, and the conditions necessary for rearing them in captivity. Several studies dealing with anatomic and taxonomic questions about the phylogenetic relation between P. patula, P. columellaris and P. pansa have been published (Wellington and Kuris 1983, Castillo-Rodriguez and Garcia-Cubas 1987, Kool 1993). There have also been ethnological studies concerning the traditional use of "Tyrian Purple" for dying materials by indigenous communities (Turok et al. 1988), aspects about the population biology (Hernández-Cortés and Acevedo-García 1987, Michel-Morfín et al. 2000), about the chemical composition of the colorant (Schunck 1880, Friedländer 1922, Wouters 1992), and the possible commercial exploitation of the snail for its purple dye (Rios-Jara et al. 1994, Castillo-Rodríguez and Amezcua-Linares 1992, Michel-Morfin and Chavez 2000). Many basic biological questions, however, have still to be answered. At what age and size do P. pansa reach sexual maturity and reproduces in nature? When is the reproductive season in Baja California Sur? Is there more than one reproductive season per year? How many egg capsules with how many eggs are laid per spawning season and how is the intracapsular development? Such biological information is necessary for the development of techniques for re-stocking natural populations, and possibly enabling them to recover, or at least buffer this threatened species from the effects of over exploitation. This study, which describes the spawning of P. pansa in captivity, is the first step towards understanding the reproduction of P. pansa.
Materials and methods
Three hundred adult snails (shell length: 27.61±s.d. 4.79 mm, range: 17.3-44.2 mm; total wet weight: 3.41±1.74 g; range: 0.92- 11.9 g) were collected between October and December 1999 during extreme low tides from exposed high intertidal rocks along the Pacific coast, at Playa Cerrito (23°1954N and 110°1038W). The snails were transferred to the laboratory (CICIMAR, La Paz) and randomly distributed into 10 inverted glass carboys with cut-off bottoms, each filled with 10 liters of seawater (salinity: 30-34 , daily water exchange; temperature range: 21-23ºC, 12 hours light/dark). They were fed unlimited amounts of squid, mussels, or beef heart daily.
The first egg capsules were found in February 2000. To estimate the duration of the deposition period newly laid capsules were counted daily and the date was marked on the outside of the carboys. To estimate the size and number of eggs per capsule a few capsules of known age were removed daily. The size of the capsules and the number of eggs/embryos per capsule were determined with a Zeiss Stemi SV11 Stereomicroscope equipped with an ocular micrometer and camera lucida projection. The surface area of the nearly circular capsules was estimated from the periphery (area = p [(perifery/2 p )2 ] determined with a digital plan measuring system (Scale Master II, Calculated Industries Inc. Carson City, NV. U.S.A.). The area of the nearly circular eggs was measured using Sigma Scan software (Jandel Scientific) and the diameter was calculated from the determined area. The duration of the experiments lasted until December 2000.
Results
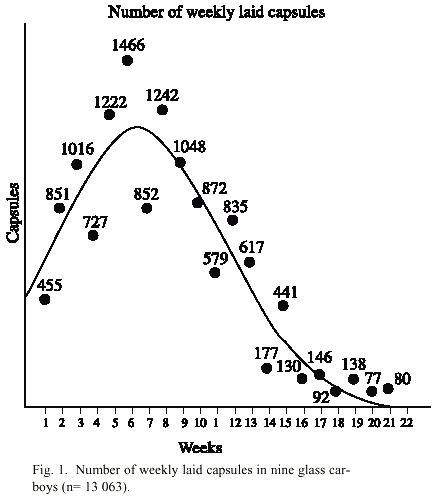
The shells of P. pansa do not show sexual dimorphism, and therefore cannot be used to determine sex. However, it was possible to observe if a penis is present or not by allowing the snail to extend undisturbed from the shell. The first egg capsules were found firmly attached to the walls of the glass bottles in February 2000. Females always deposited the egg capsules below the water surface. The peak of reproduction was between May and June. And a second peak in spawning occurred between October and December. The egg capsules of the purple snail are of lentil size and shape and are laid in a compact mass of multiple capsules (up to 30 capsules/deposition/ female) firmly attached to the glass walls of culture vessels. Capsules were added daily to the periphery of previously deposited capsules, so that egg clusters with hundreds of capsules of different ages and spawned by different females were eventually formed. All ten carboys contained a large number of egg capsules. During a period of three months, 15 females deposited nearly 2 000 capsules on the wall of only one carboy (Fig. 1).
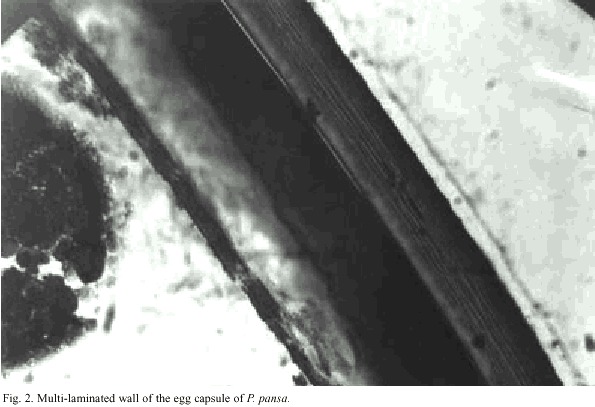
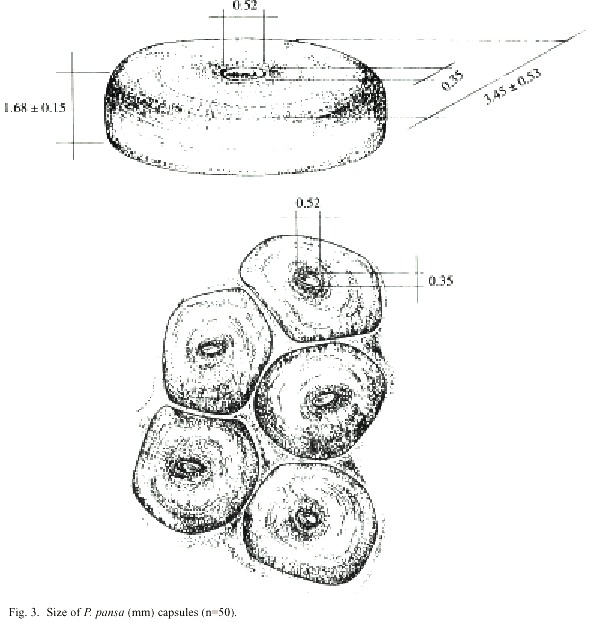
Freshly spawned egg capsules had a gelatinous covering that hardened during the first hours of development. Immediately after oviposition, capsule walls were white, moderately translucent and the eggs inside were clearly visible, and very difficult to remove. With aging, the color of the capsules changed from yellowish to coffee brown, and became easy to remove from the glass walls. After hardening the egg coverings were thick walled and composed of multiple laminae. Sutures could not be detected. Capsules containing non-viable embryos turned purple. (Fig. 2)The capsules (n=50) measured about 1.7 mm in height (1.66±s.d. 0.32 mm), 4.36±0.73 mm in length, and 3.34±0.78 mm in width. The oval escape aperture (length 0.52 mm, width 0.35 mm) is placed centrally on top of the egg capsules. On average each capsule contained 436 eggs (±s.d. 213.6, n=50, range 95-1092). The oval eggs had a diameter of 188 µm (±s.d.13,0 n=50, range 163.1-224.6 µm). (Fig. 3).
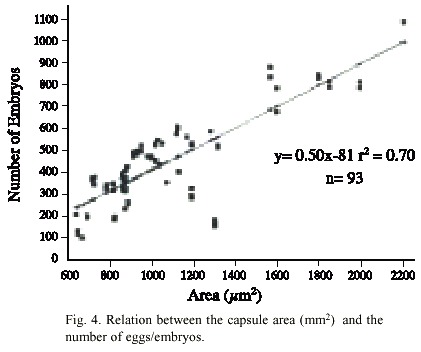
A close relationship was observed between the size of the capsules and the number of eggs/embryos: The larger the capsule the greater number of eggs/embryos it contained (Fig. 4).
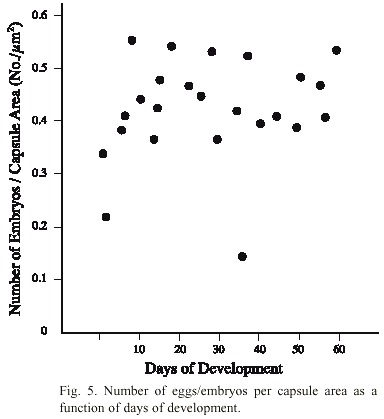
The size of the capsules increased only slightly as embryos developed and the velar cilia became active. Therefore the number of eggs/embryos per capsule area versus the days of development remained unchanged (Fig. 5).
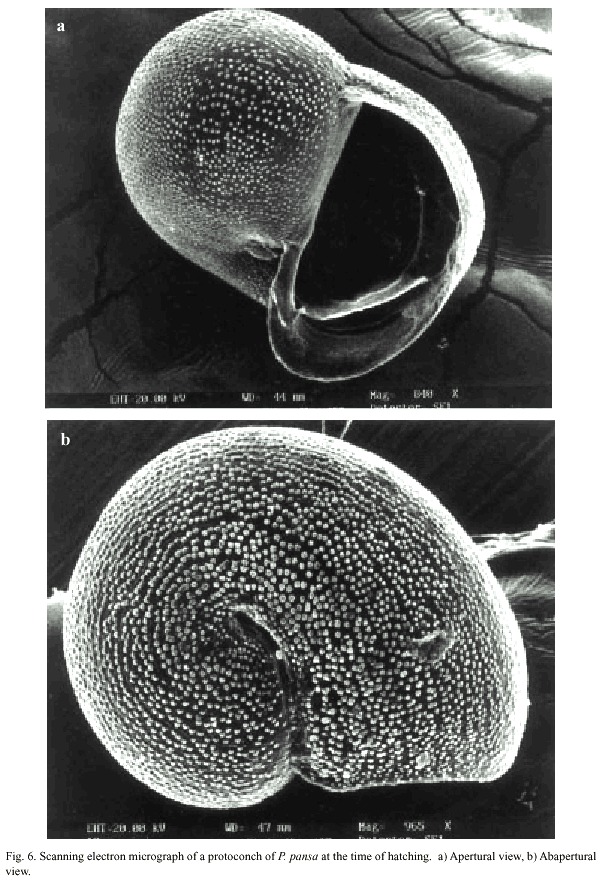
The intracapsular development to a free-swimming veliger larva took six to eight weeks at 22-23ºC and hatching occurred among capsules non-synchronously. At the time of hatching torsion was already completed so that the larvae were able to retract completely into the shell. The shell length of recently hatched veligers was 288.1 µm (±s.d 7.33 n= 20, range 285.7-309.5). The shell appeared translucent and the internal organs were visible (Fig. 6).
When the veliger larva hatched the velum was bilobed, used for locomotion and feeding, and was translucent without spots or stripes. With the help of the vela recently hatched animals immediately collected microalgae.
Discussion
Purple snails were collected between October and December, and the first egg capsules were laid in the laboratory February in the laboratory. During this period copulation was not observed. Ramorino (1975, 1979) reported that females of the Chilean muricid Concholepas concholepas can store viable sperm in the seminal receptacles for several months after copulation. Therefore it is possible that copulation of P. pansa occurred months before fertilization and spawning. Females always deposited egg capsules below the water surface. A similar behaviour was described by Lewis (1960) for Thais patula (= P. patula), which laid the egg capsules below the low water mark, usually beneath flat stones. Higher caenogastropods enclose fertilized ova in proteinaceous capsules that are molded into morphologically distinct structures of varying complexity. These distinctive capsule morphologies are attributable to specific genera or families (DAsaro 1986). Muricid egg capsules have species-specific morphologies. Specific characteristics include shape and sculpture with the latter being most distinctive (DAsaro 1991). The egg capsules of P. pansa, to my knowledge, have not previously been described in detail. Lewis (1960) described the egg capsules of P. patula as flat and rounded, and up to about 4 mm in width egg capsules – a description very similar in size and shape to the capsules of P. pansa. The description of the egg capsules given by Kool (1988) of the congener P. columellaris is completely different. Kool (1993) explained this by the fact, that his descriptions were based on specimens that were collected without the animal that laid them, and are probably based on capsules of a different species. DAsaro (1991) reports that all egg capsules from the more than 80 muricid species from the Thorson Collection, had sutures in a vasiform pattern. It is interesting to note that the capsules of P. pansa do not appear to have sutures. The egg capsules of P. patula, as sketched by Lewis (1960) also seem to have also no sutures.As a protection against biological and physical stresses, the capsules of marine snails are covered with multilaminated tough fibers (Strathmann 1987, Rawlings 1995, 1999). The egg capsules of P. pansa are thick-walled and composed of multiple laminae, possibly as a protection against the harsh environmental conditions in the intertidal zone. In most invertebrates capsules swell as the embryos develop, particularly after velar cilia become active (Strathmann 1987). In our studies with the thick-walled capsules of P. pansa this finding could not be confirmed with certainty.
Freshly spawned egg capsules were white, moderately translucent with the eggs inside clearly visible. With aging, the color of the capsules changed from yellowish to coffee brown. Capsules containing non-viable embryos turned purple. Gallardo (1973), as well as Riquelme and Chavez (1991), noted that the development of purple color in non-viable capsules of the muricid C. concholepas was due to bacterial colonization. Also DAsaro (1966) reported from T. haemastoma floridana that dark purple capsules contained dead embryos. This contradicts the observation by Turok et al. (1988), who reported that the change of color to purple was due to embryonic maturation.
In view that most principal life-history features of P. pansa are yet unknown, a comparison with other intertidal muricids could give some indications about the generality of our results. The close relative of P. pansa with lecitotrophic intracapsular development is the Chilean intertidal snail C. concholepas. The eggs of this muricid measure 147-170 µm (Ramorino 1979), a size very similar to P. pansa (188 µm). The intracapsular development of C. concholepas takes 36-37 days at a water temperature of 16.6 – 18.7°C (Gallardo 1973) and 60-84 days at 12-14°C (Ramorino 1975) until the hatching of 250 µm veliger larva (DiSalvo 1988). At 22-23°C the intracapsular development of P. pansa took 50 to 60 days until hatching of a 288 µm veliger larva. As Spight et al. (1974) described, most developmental patterns are directly dependent on egg size, and the intracapsular development time of intertidal muricids with lecitotrophic development is relatively long. Pre-hatching period and hatching size are directly correlated with egg size, while the length of the pelagic period is inversely related to it (Gallardo and Perron 1982). Species with a planktotrophic larval stage produce more numerous small eggs and numerous planktotrophic larvae, than species with direct development (Thorson 1950, Gallardo and Perron 1982).In our experiments one female snail of P. pansa produced more than 50 000 eggs during one spawning season. As Thorson (1950) and Spight (1976, 1977) described, benthic marine organisms with a large number of small eggs undergo a long period of intracapsular development, and most muricids in tropical rocky shore areas hatch as veligers. This finding could be confirmed for P. pansa, where the large number of small eggs in each capsule underwent an intracapsular development for 6-8 weeks until fully developed, small size veliger larva hatched.
The maintenance of egg capsules from P. pansa under laboratory conditions was difficult, due to the high frequency of contamination of the egg capsules with predators, like copepods and polychaetes. Nearly sterile rearing conditions combined with frequent water changes were necessary to assure survival of the capsules.
In experiments with C. concholepas, it took many years and much efforts until the first successful larval rearing, metamorphosis and settlement of larvae of this species could be reported (DiSalvo 1988, DiSalvo and Carriker 1994). Similar hurdles have to be overcome in rearing the larvae of the purple snail, but now after the successful spawning and intracapsular development of P. pansa the first basic steps have been made towards larval rearing in the laboratory.
Acknowledgments
We thank M. Ortiz for his help in taking care of the adult snails and counting daily the egg capsules and the eggs and embryos in them. Thanks to C. Caceres for the size determination of P. pansa eggs. Special thanks to P. Middelfart (Australian Museum, Sydney) for the scanning photographs of the protoconchs, and to O. Armendariz for his help with the figures. I thank the two anonymous reviewers for helpful suggestions and constructive criticisms of an earlier draft. This work was supported by a grant (No. 31566-N) from CONACYT, Mexico, and by a grant from COFAA and EDI to the author.
Resumen
Se describe el desove del gasterópodo Plicopurpura pansa (Gould, 1853) (Gastropoda, Prosobranchia, Muricidae) a 22-23°C en el laboratorio. Cada hembra depositó diariamente de 1 a 20 cápsulas por lo menos durante 20 semanas y produjo hasta 150 cápsulas durante la estación de reproducción. Durante el tiempo de desove se formaron agrupaciones de cientos de cápsulas de diferentes edades y diferentes hembras. Cada cápsula contenía, según del tamaño, en promedio 436 embriones (± s.d. 213.6, ámbito: 95-1 092, n=50). Los embriones se desarrollaron sin huevos nutricios. Después de seis a ocho semanas del desarrollo lecitotrófico intracapsular eclosionaron larvas veliger planctotróficas completamente desarrolladas con dos velum de forma lobulada.
Referencias
Anónimo 1988. Diario Oficial de la Federación. Organo del Gobierno Constitucional de los Estados Mexicanos. Secretaría de Pesca. March 30, 1988 p. 10-12. [ Links ]
Born, W. 1936. Purpur bei den Indianern Mittelamerikas –ein Erbe der Antike. Ciba Rundschau 4: 130-134. [ Links ]
Castillo-Rodriguez, Z.G. & A. Garcia-Cubas. 1987. Morfología y anatomía del caracol "morado" Purpura spp. en las costas de México. pp. 311-321. In Memorias Volume 3, Sociedad de Malacología, México. [ Links ]Castillo-Rodríguez, Z.G. & F. Amezcua-Linares. 1992. Biología y aprovechamiento del caracol morado Plicopurpura pansa (Gould, 1853) (Gastropoda: Neogastropoda) en la costa de Oaxaca, México. Anal. Inst. Cien. Mar y Limnol., U. Nac. Autónoma
de México 19(2): 223-234.
Clench, W.J. 1947. The genera Purpura and Thais on the Western Atlantic. Johnsonia 2(23): 61-91. [ Links ]
DAsaro, C.N. 1966. The egg capsules, embryogenesis, and early organogenesis of a common oyster predator, Thais haemasoma floridana (Gastopoda: Prosobranchia). Bull. Mar. Sci. 1(4): 884-914. [ Links ]
DAsaro, C.N. 1986. Egg capsules of eleven marine proso-branchs from northwest Florida. Bull. Mar. Sci. 39(1): 76-91. [ Links ]
DAsaro, C.N. 1991. Gunnar Thorsons world-wide collection of prosobranch egg capsules: Muricidae. Ophelia 35(1): 1-101. [ Links ]
DiSalvo, L.H. 1988. Observations on the larval and post-metamorphic life of Concholepas concholepas (Bruguière, 1789) in laboratory culture. The Veliger 30(4): 358-368. [ Links ]
DiSalvo, L.H. & M.R. Carriker. 1994. Planktonic, metamorphic, and early benthic behavior of the Chilean loco Concholepas concholepas (Muricidae, Gastropoda, Mollusca). J. Shellfish Res. 13(1): 57-66. [ Links ]
Friedländer, P. 1922. Über die Farbstoffe aus Purpura aperta und Purpura lapillus. Ber. Deut. Chem. Ges. 55: 1655-1658. [ Links ]
Gallardo, C. 1973. Desarrollo intracapsular de Concholepas concholepas (Brugière) (Gastropoda, Muricidae). Museo Nacional de Historia Natural. Santiago de Chile. Pub. Ocas. 16 p. [ Links ]
Gallardo, C.S. & F.E. Perron. 1982. Evolutionary ecology of reproduction in marine benthic molluscs. Malacologia 22(1-2): 109-114. [ Links ]
Gerhard, P. 1962. Shellfish dye in America. pp. 177-191. In Proc. Vol. III. 35th. Intern. Congr. Americanists, Mexico City 1962. Editorial de Libros, México, D.F. (Imprint 1964). [ Links ]
Hernández-Cortés, E. & J. Acevedo-García. 1987. Aspectos poblacionales y etnobiológicos del caracol Purpura pansa, Gould, 1853 en la costa de Oaxaca. Tesis de Licenciatura en Biología. Universidad Nacional Autónoma de México, Facultad de Ciencias. 147 p. [ Links ]
Jackson, J.W. 1917. The geographical distribution of the shell-purple industry. pp. 1-29. In J.W. Jackson (ed.). Shells as evidence of the migrations of early culture. University, Manchester, England. [ Links ]
Keen, A.M. 1971. Sea shells of tropical West America: Marine molluscs from Baja California to Peru. Stanford U. Stanford, CA. 1064 p. [ Links ]
Kool, S. P. 1988. Aspects of the anatomy of Plicopurpura patula (Prosobranchia: Muricoidea: Thaidinae), new combination, with emphasis on the reproductive system. Malacologia 29(2): 373-382. [ Links ]
Kool, S.P. 1993. Phylogenetic analysis of the Rapaninae (Neogastropoda: Muricidae). Malacologia 35(2): 155-259. [ Links ]
Lewis, J.B. 1960. The fauna of rocky shores of Barbados, West Indies. Can. J. Zool. 38: 391-435. [ Links ]
Martens, v. E. 1874. Purpur und Perlen. Sammlung gemeinverständlicher Vorträge 9(214): 1-55. [ Links ]
Martens, v. E. 1898. Purpur-Färberei in Central-America. Z. Ethnol. 30: 482-486. [ Links ]
Michel-Morfin, J.E. & E A.Chávez. 2000. Effect of repetitive dye extraction over yield and survival rate of the purple snail Plicopurpura pansa (Gould, 1853). J. Shellfish Res. 9: 913-917. [ Links ]
Michel-Morfín, J.E., E.A. Chávez & V. Landa. 2000. Population parameters and dye yield of the purple snail Plicopurpura pansa (Gould, 1853) of West Central Mexico. J. Shellfish Res. 19: 919-925. [ Links ]
Nutall, Z. 1909. A curious survival in Mexico of the use of the Purpura shell-fish for dyeing. pp. 366-384. In Putman Anniversary Volume, Cedar Rapids, Iowa. [ Links ] Paredes, C., P. Huamán, F.Cordoso, R.Vivar & V.Vera. 1999. Estado actual del conocimiento de los moluscos acuáticos en el Perú. Rev. Peruana Biol. 6(1): 5-47. [ Links ]
Peña, G.G.M. 1970. Zonas de distribución de los gasterópodos marinos del Perú. Anal. Científicos U. Nac. Agraria 8: 153-170. [ Links ]
Ramorino, L. 1975. Ciclo reproductivo de Concholepas concholepas en la zona de Valparaíso. Rev. Biol. Mar. 15(2): 149-177. [ Links ]
Ramorino, L. 1979. Conocimiento científico actual sobre reproducción y desarrollo de Concholepas concholepas (Mollusca: Gastropoda: Muricidae). Biol. Pesquera Chile 12: 59-70. [ Links ]
Rawlings, T.A. 1995. Direct observation of encapsulated development in muricid gastropods. The Veliger 38(1): 54-60. [ Links ]
Rawlings, T.A. 1999. Adaptations to physical stresses in the intertidal zone: The egg capsules of neogastropod molluscs. Amer. Zool. 39: 230-243. [ Links ]
Rios-Jara, E., H.G. León-Alvarez, L. Lizárraga-Chávez & J.E. Michel-Morfín. 1994. Producción y tiempo de recuperación del tinte de Plicopurpura patula pansa (Neogastropoda: Muricidae) en Jalisco, México. Rev. Biol. Trop. 42(3): 537-545. [ Links ]
Riquelme, C.E. & P.C. Chavez. 1991. Colonization of vibrios on developmental stages of Concholepas concholepas (Bruguière, 1789), (Mollusca: Muricidae). pp. 84-95. In K. Koop (ed), Ecology of marine aquaculture. A work-shop on research in aquaculture. Osorno, Chile, 1991. Intern. Found. Science, Stockholm, (Imprint 1994). [ Links ]
Schunck, E. 1880. LII. Notes on the purple of the ancients (continuation). 3. Purple dyeing in modern times. J. Chem. Soc. 37: 613-617. [ Links ]
Spight, T.M. 1976. Ecology of hatching size for marine snails. Oecologia (Berlin) 24: 283-294. [ Links ]
Spight, T.M. 1977. Latitude, habitat, and hatching type for muricacean gastropods. Nautilus 91(2): 67-71. [ Links ]
Spight, T.M., C. Birkeland & A. Lyons. 1974. Life histories of large and small murexes (Prosobranchia: Muricidae). Mar. Biol. 24: 229-242. [ Links ]
Strathmann, M.F. 1987. Phylum Mollusca, Class Gastropoda, Subclass Prosobranchia. Chapter 11. pp. 220-267. In M.F. Strathmann. Reproduction and development of marine invertebrates of the Northern Pacific Coast. Data, and methods for the study of eggs, embryos and larvae. U. Washington, Seattle and London, 670 p. [ Links ]
Thompson, J. 1994. Shellfish purple: the use of Purpura patula pansa on the Pacific coast of Mexico. Dyes in History and Archaeology 13: 3-6. [ Links ]
Thorson, G. 1950. Reproductive and larval ecology of marine bottom invertebrates. Biol. Rev. 25: 1-45. [ Links ]
Turok, M. 1999. El carcol Purpura pansa: Un pequeño gran recurso. Programa Cultural Tierra Adentro. http://www.cnca.gob.mx//cnca/buena/descentra/tierra/ mturok.html 8 p. [ Links ]
Turok, M., A. Sigler-M., E. Hernández-C., J. Acevedo-G., R. Lara-C. & V. Turcott. 1988. El caracol púrpura. Una tradición milenaria en Oaxaca. Secretaria de Educación Publica. Dirección General de Culturas Populares. México, D.F. 164 p. [ Links ]
Wellington, G.M. & A.M. Kuris. 1983. Growth and shell variation in the tropical eastern Pacific intertidal gastropod genus Purpura: Ecological and evolutionary implications. Biol. Bull. 164: 518-535. [ Links ]
Wouters, J. 1992. A new method for the analysis of blue and purple dyes in Textiles. Dyes in History and Archaeology 10: 17-21. [ Links ]