Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.51 n.3-4 San José Sep. 2003
from Monteverde, Costa Rica
Mary C. Setzer 1, Debra M. Moriarity 1, Robert O. Lawton 1, William N. Setzer * 2, Glenn A. Gentry 3, & William A. Haber 4
1 Department of Biological Sciences, The University of Alabama in Huntsville, Huntsville, Alabama 35899, U.S.A
2 Department of Chemistry, The University of Alabama in Huntsville, Huntsville, Alabama 35899, U.S.A.
3 Department of Microbiology, University of Mississippi Medical Center, Jackson, Mississippi 39216, U.S.A.
4 Apdo. 50-5655, Monteverde de Puntarenas, Costa Rica, Central America; Missouri Botanical Garden, St. Louis, Missouri 63110, U.S.A.
*To whom correspondence should be addressed. Phone: 256-824-6519. Fax: 256-824-6349; wsetzer@chemistry.uah.edu
Received 13-XII-2000. Corrected 06-VI-2001. Accepted 14-I-2002.
Abstract
A pharmacological survey of plants from Monteverde, Costa Rica, including 165 species representing 61 families has been carried out. Crude plant extracts have been tested for in-vitro bactericidal and fungicidal activity as well as cytotoxic and anti-herpes activity. Of these, 123 extracts exhibited notable cytotoxicity, 62 showed antibacterial activity, 4 showed antifungal activity, and 8 showed promising antiviral activity. Thus, 101 of the plant species examined in this work, or 62%, showed marked bioactivity in one or more bioassays. These results underscore the phytomedicinal potential of Neotropical cloud forests.
Key Words: Antibacterial activity, antifungal activity, antiherpes activity, cytotoxic activity, Monteverde Cloud Forest Preserve, Costa Rica.
The pharmaceutical value of tropical forests is well known. Some 57% of presciption drugs sold in the United States are based on natural products, that is, are either natural products themselves, or are synthetic derivatives or analogs of natural products (Grifo et al. 1997). This represents retail sales in 1990 of approximately $80 billion (Artuso 1997). From 1983 to 1995, more than 60% of new approved drugs for treatment of cancer and infections were based on natural products (Cragg et al. 1997). It has been estimated that the economic value of each undiscovered drug from a tropical plant is $449 million (Mendelsohn and Balick 1995). Neotropical forests therefore represent a stockpile of immense economic as well as medicinal potential. In collaboration with the Centro Cientifico Tropical, which owns and administers the Monteverde Cloud Forest Preserve, we have been interested in the pharmaceutical potential of Costa Rican plants (Setzer 2000, Setzer et al. 1998, 1999, 2000ab, 2001). In this paper, we report the collection, extraction, and bioactivity screening of a number of higher plants from the Monteverde region, Costa Rica. This work complements our earlier screening survey of the Araliaceae from Monteverde (Setzer et al. 1992) and greatly expands the scope of our survey.
The Monteverde region of the central Cordillera de Tilarán in northwestern Costa Rica is, like most tropical montane areas, physiographically and climatically diverse (Clark et al. 2000). This environmental diversity results in an extraordinarily high between-site component of biodiversity; disjunct patches of tropical dry forest occupy edaphically dry narrow ridges on the upper Pacific slope only 4 km from true lower montane rain forests along the crest of the Cordillera (Clark et al. 2000, Haber 2000). Consequently the region is among the floristically most diverse in the world. The slopes of the Cordillera above 1200 m elevation contain ~1700 plant species – roughly the number in the La Selva Biological Station in the Caribbean lowlands of Costa Rica, or in the floodplains and upland terraces along the Rio Manú in the Amazonian lowlands of Peru – while the area above 700 m in the Cordillera de Tilarán contains ~3000 plant species.
This plant diversity suggests great phytomedicinal potential. Although the upper San Luis valley and the Monteverde-Cerro Plano-Santa Elena area on the upper Pacific slope of the Cordillera were occupied by Amerindian agriculturalists in Pre-Columbian times (Timm 2000, Sheets and McKee 1994), the cloud forests above 1500 m were apparently never settled. At any rate, following the Spanish conquest, the original inhabitants disappeared, and their ethnobotanical knowledge was lost. Consequently we have focused our survey upon plant groups of known utility in other parts of the world, hoping to encounter novel compounds and biological activities from phytochemically rich taxa. In addition we have sampled plants with characteristics, such as strongly aromatic leaves or bark, that suggest the presence of potentially interesting compounds.
Materials and methods
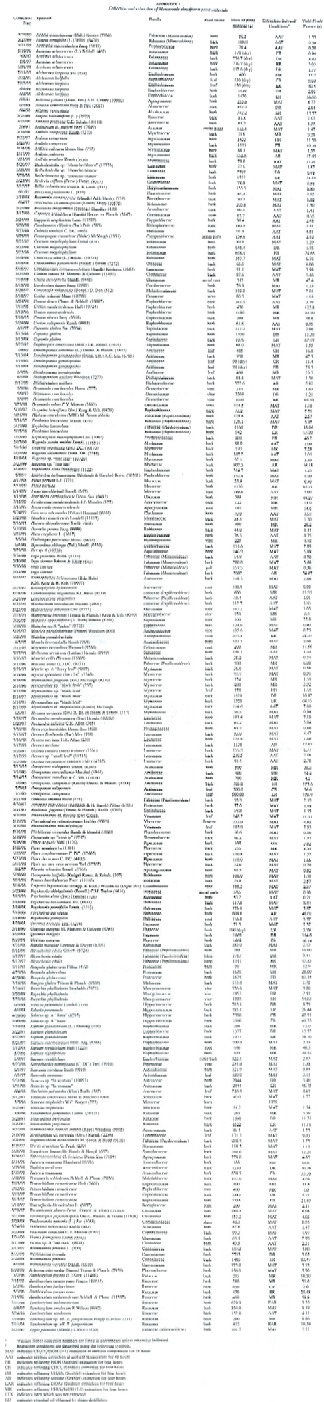
Study Area, Collection and Extraction of Plant Materials. Plant material was collected from the Monteverde Cloud Forest Reserve (ca. 1600 m elevation), the community of Monteverde (ca. 1400 m elevation), or from the San Luis Biological Station (ca. 700 m elevation). Plant identification was carried out by either R. O. Lawton or W. A. Haber by comparison to specimens at the Missouri Botanical Garden. All voucher specimens are currently at the Missouri Botanical Garden. Plant materials were collected, chopped, and immediately extracted (Appendix 1). Extracts for initial screening were obtained by infusion of approximately 100 g of finely chopped plant material in either a mixture of ethanol/chloroform (1:1) or in acetone at ambient temperature for 48 hr. Plant materials which yielded extracts showing activity in initial screening were then collected in amounts of 1-2 kg. The chopped plant materials were extracted with either dichloromethane or chloroform for 4 hr, followed by a second extraction with ethanol for 4 hr; or by Soxhlet extraction with refluxing acetone for 4 hr (see Appendix 1). The extract solutions were concentrated by rotary evaporation to give the crude extracts which were stored at –30°C prior to screening. Evaporated extracts were prepared for screening by making 1% w/w solutions in dimethylsulfoxide (DMSO).
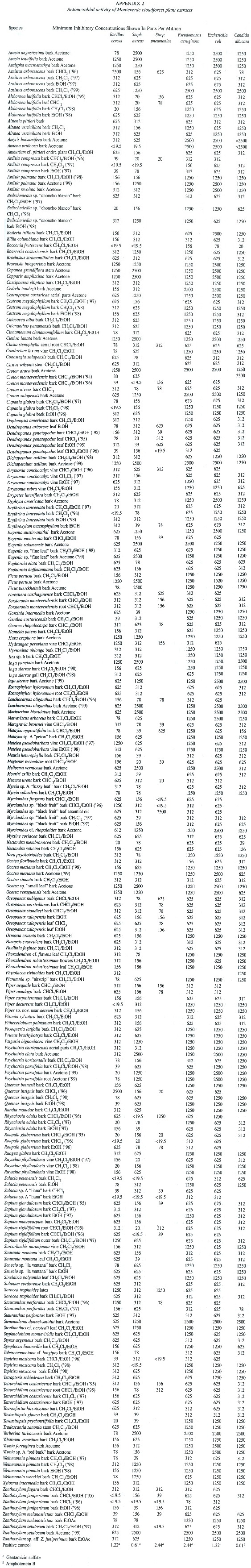
Antimicrobial Screening. Crude extracts were screened for antimicrobial activity against Gram-positive bacteria, Bacillus cereus (ATCC No. 14579), Staphylococcus aureus (ATCC No. 29213), Streptococcus pneumoniae (ATCC No. 6303); Gram-negative bacteria, Pseudomonas aeruginosa (ATCC No. 27853) and Escherichia coli (ATCC No. 25922). Minimum inhibitory concentrations (MIC) were determined using the microbroth dilution technique (Sahm and Washington 1991). Dilutions of the crude extracts were prepared in cation-adjusted Mueller Hinton broth (CAMHB) beginning with 50 µl of 1% w/w solutions of crude extracts in DMSO plus 50 µl CAMHB. The extract solutions were serially diluted (1:1) in CAMHB in 96-well plates. Organisms at a concentration of approximately 1.5 x 108 colony forming units (CFU)/ml were added to each well. Plates were incubated at 37°C for 24 hr; the final minimum inhibitory concentration (MIC) was determined as the lowest concentration without turbidity. Gentamicin was used as a positive antibiotic control; DMSO was used as a negative control. Antifungal activity was determined as described above using Candida albicans (ATCC No.10231) in yeast-nitrogen base growth medium with approximately 7.5 x 107 CFU/ml. Amphotericin B was the positive control. Antimicrobial results are listed in Appendix 2.
Cytotoxicity Screening. Human Hep G2 hepatocellular carcinoma cells (ATCC No. HB-8065) (Knowles et al. 1980) were grown in an air environment at 37°C in Dulbeccos Modified Eagles Medium (DMEM) with L-glutamine and 1000 mg glucose per liter of medium, supplemented with 100.000 units penicillin and 10.0 mg streptomycin per liter of medium, and buffered with 30mM N-(2-hydroxyethyl) piperazine-N-2-ethane-sulfonic acid (Hepes), pH 7.35. Cells were plated using medium supplemented with 10% fetal bovine serum and maintained between passaging using medium supplemented with 10% horse serum and 5% fetal bovine serum.
Rat H-4-II-E hepatoma cells (ATCC No. CRL-1548) (Pitot et al. 1964) were grown in an air environment at 37°C in DMEM with L-glutamine and 1000 mg glucose per liter of medium, supplemented with 10% fetal bovine serum, 100.000 units penicillin and 10.0 mg streptomycin per liter of medium, and buffered with 30mM Hepes, pH 7.35.
Human MDA-MB-231 breast adenocarcinoma cells (ATCC No. HTB-26) (Cailleau et al. 1974) were grown in an air environment at 37°C in Leibovitzs L-15 medium with L-glutamine, supplemented with 10% fetal bovine serum, 100,000 units penicillin and 10.0 mg streptomycin per liter of medium, and buffered with 30mM Hepes, pH 7.35.
Human Hs 578T breast ductal carcinoma cells (ATCC No. HTB-129) (Hackett et al. 1977) were grown in a 3% CO2 environment at 37°C in DMEM with 4500 mg glucose per liter of medium, supplemented with 10% fetal bovine serum, 10 µg bovine insulin, 100.000 units penicillin and 10.0 mg streptomycin per liter of medium, and buffered with 44 mM NaHCO3 , pH 7.35.
Human 5637 primary bladder carcinoma cells (ATCC No. HTB-9) (Fogh 1978) were grown in a 3% CO 2 environment at 37°C in RPMI-1640 medium with L-glutamine, supplemented with 10% fetal bovine serum, 100.000 units penicillin and 10.0 mg streptomycin per liter of medium, and buffered with 28 mM NaHCO3 , pH 7.35.
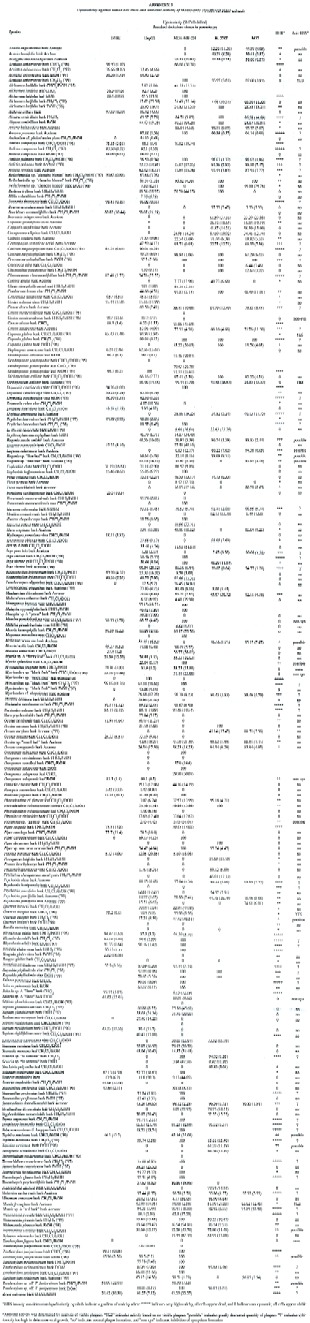
Hep G2 cells were plated into 96-well cell culture plates, at 1.8 x 104 cells per well; H-4-II-E cells at 2.3 x 103 cells per well; MDA-MB-231 cells at 2.6 x 104 cells per well; Hs 578T cells at 1.0 x 105 cells per well; and 5637 cells at 5.1 x 103 cells per well. The volume in each well was 100µl for all cell types. After 48 h, supernatant fluid was removed by suction and replaced with 100 µl growth medium containing 2.5 µl of DMSO solution of extracts or compounds (1% w/w in DMSO), giving a final concentration of 250 µg/ml for each extract or compound. Solutions were added to wells in four replicates. Medium controls and DMSO controls (25 µl DMSO/ml) were used. Tingenone (250 µg/ml) was used as a positive control (Setzer et al. 1998). After the addition of compounds, plates were incubated for 48 hr at 37°C; medium was then removed by suction, and 100 µl of fresh medium was added to each well. In order to establish percent kill rates, the CellTiter 96Ò AQueous Non-Radioactive Cell Proliferation Assay was performed (Anonymous 1996). After colorimetric readings were recorded (using a Molecular Devices SpectraMAX Plus microplate reader, 490 nm), average absorbances, standard deviations, and percent kill ratios (%killcmpd /%killDMSO ) were calculated (Appendix 3).
Antiviral Screening. Baby hamster kidney (BHK) cells (Russell 1962) were grown in a 5% CO2 environment at 37°C in DMEM with L-glutamine and 4500 mg glucose per liter of medium, supplemented with 80 mg gentamicin sulfate, 5% fetal bovine serum, and 8.8 ml 200 mM L-glutamine per liter of medium, and buffered with 44 mM NaHCO3 , pH 7.35.
Extracts were screened for activity against Herpes simplex virus type 1 by a modification of the plaque reduction assay (Gentry and Aswell 1975; Aswell et al. 1977). The anti-HSV assay was carried out in confluent BHK monolayers in 24-well cell culture plates. The supernatant fluid was removed, wells were inoculated with 30-60 plaque-forming units (PFU) in 0.2 ml culture medium and the plates incubated for 2 hr with continuous slow agitation. The supernatant fluid was removed and 1.0 ml culture medium was added to each well, followed by 10 µl of 1% solution of the crude extract in DMSO. Virus controls were provided by the addition of 10 µl DMSO alone, or 10 µl medium to inoculated wells. The plates were incubated for 30 min with intermittent agitation and incubated further for 48 hr. Plaques were counted and qualitative cytotoxicity was assessed using an inverted microscope, after 24 and 48 hr, as described in Appendix 3.
Results
Plant extraction conditions and yields are summarized in Appendix 1. Antimicrobial activities are presented in Appendix 2. Cytotoxicity data and antiviral activity are compiled in Appendix 3. A total of 165 plant species from 61 families were collected, extracted, and screened in the biological assays described above. Of these, 62% have shown remarkable biological activity in at least one of the assays. Notable cytotoxic activity was considered to be > 90% killing at 250 µg/ml, this was found in 123 of the extracts screened. MIC values of < 40 µg/ml in the microbroth dilution assay were considered to be antimicrobial, 62 extracts exhibited this activity. Eight extracts were found to be antiviral, showing no viral plaque formation with little or no cytotoxic activity against the host cells at 100 µg/ml.
Discussion
Several families have proven to be promising sources of biological activity in this study. In the Clusiaceae, 100% of those species tested showed activity in at least one of the biological assays, 89% of the Myrtaceae, 86% of the Rutaceae, 83% of the Araliaceae, and 54% of the Fabaceae species studied exhibited activity. Members of these families are used in traditional medicine throughout their native environments. Work is currently underway in our laboratories to continue collection and screening of higher plants from the Monteverde region, and to isolate, purify, and identify the active components in these plant extracts.
Acknowledgments
Support for this work was provided in part by grants from the National Institutes of Health (Grant Nos. 1 R15 GM46120-01A1 and 1 R15 CA74343-01). We are very grateful to the Monteverde Cloud Forest Preserve and the Tropical Science Center for granting us permission to collect plant materials from the Preserve. We are grateful to Maynor Vargas Arguedas for permission to collect plant materials on the property of Hotel El Bosque, Monteverde. We thank Susan M. Fields, Jennifer M. Schmidt, Michael T. Holland, Ginger K. Lehrmann, and Suzanne M. Morcomb, for assistance with biological screening, and Amanda L. Hopper, Sharon M. Talley, Stephen J. Fuemmeler, Amanda D. Jones, Gabrielle A. Ehinger, and Nichole J. Wangbickler, for helping with plant collection and extraction in Monteverde. We are grateful to Research Genetics, Inc., for their generous gift of the 96-well plate reader.
Resumen
Se realizó un análisis farmacológico de plantas de Monteverde, Costa Rica, que incluye 165 especies representantes de 61 familias. Se probó in-vitro la actividad bactericida y fungicida, así como la actividad citotóxica y anti-herpes de extractos crudos de plantas. De estos, 123 extractos exhibieron una notable citotoxicidad, 62 mostraron actividad antibacterial, 4 presentaron actividad antihongos, y 8 mostraron una promisoria actividad antiviral. Así, de las 101 especies de plantas examinadas en este trabajo, 62% presentaron una marcada actividad biológica en uno o más de los bioensayos. Estos resultados subrayan el potencial fitomédico de los bosques nubosos Neotropicales.
References
Anonymous, 1996. Technical Bulletin #245. CellTiter 96® AQueous one solution cell proliferation assay. Promega, Madison, Wisconsin. [ Links ]
Artuso, A. 1997. Chapter 8, pp. 184-204. In F. Grifo & J. Rosenthal (eds.). Biodiversity and human health. Island, Washington DC. [ Links ]
Aswell, J.F., G.P. Allen, A.T. Jamieson, D.E. Campbell & G.A. Gentry. 1977. Antiviral activity of arabinosylthymine in herpesviral replication: mechanism of action in vivo and in viro. Antimicrob. Agents Chemother. 12: 243-254. [ Links ]
Cailleau, R., R. Young, M. Olive & W.J. Reeves, Jr. 1974. Breast tumor cell lines from pleural effusions. J. Natl. Cancer Inst. 53: 661-674. [ Links ]
Clark, K.L, R.O. Lawton & P.R. Butler. 2000. The physical environment. pp. 15-38. In N.M. Nadkarni & N.T. Wheelwright (eds.). Monteverde: Ecology and conservation of a tropical cloud forest. Oxford, New York. [ Links ]
Cragg, G.M., D.J. Newman & K.M. Snader. 1997. Natural products in drug discovery and development. J. Nat. Prod. 60: 52-60. [ Links ]
Fogh, J. 1978. Cultivation, characterization, and identification of human tumor cells with emphasis on kidney, testis, and bladder tumors. Natl. Cancer Inst. Monogr. 49: 5-9. [ Links ]
Gentry, G.A. & J.F. Aswell. 1975. Inhibition of herpes simplex virus replication by araT. Virology 65: 294-296. [ Links ]
Grifo, F., D. Newman, A.S. Fairfield, B. Bhattacharya & J.T. Guppenhoff. 1997. Chapter 6, pp. 131-163. In F. Grifo & J. Rosenthal (eds.). Biodiversity and human health. Island, Washington DC. [ Links ]
Haber, W.A. 2000. Plants and vegetation, pp. 39-94. In N.M. Nadkarni & N.T. Wheelwright (eds.). Monteverde: Ecology and conservation of a tropical cloud forest. Oxford, New York. [ Links ]
Haber, W.A., W. Zuchowski & E. Bello. 2000. An introduction to cloud forest trees: Monteverde, Costa Rica. Mountain Gem, Monteverde, Costa Rica. [ Links ]
Hackett, A.J., H.S. Smith, E.L. Springer, R.B. Owens, W.A. Nelson-Rees, J.L. Riggs & M.B. Gardner. 1977. Two syngeneic cell lines from human breast tissue: the aneuploid mammary epithelial (Hs578T) and the diploid myoepithelial (Hs578Bst) cell lines. J. Natl. Cancer Inst. 58: 1795-1806. [ Links ]
Knowles, B.B., C.C. Howe & D.P. Aden. 1980. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science 209: 497-499. [ Links ]
Mendelsohn, R. & M.J. Balick. 1995. The value of undiscovered pharmaceuticals in tropical forests. Econ. Bot. 49: 223-228. [ Links ]
Pitot, H.C., C. Peraino, P.A. Morse & V.R. Potter. 1964. Hepatomas in tissue culture compared with adapting liver in vivo. Natl. Cancer Inst. Monogr. 13: 229-245. [ Links ]
Russell, W.C. 1962. A sensitive and precise plaque assay for herpes virus. Nature (London) 195: 1028-1029. [ Links ]
Sahm, D.H. & J.A. Washington. 1991. Antibacterial susceptibility tests: Dilution methods. In A. Balows, W.J. Hausler, K.L. Herrmann, H.D. Isenberg & H.J. Shamody (eds.). Manual of clinical microbiology. American Society for Microbiology, Washington DC. [ Links ]
Setzer, W.N. 2000. The search for medicines from the plants of Monteverde, pp. 452-453. In N.M. Nadkarni & N.T. Wheelwright (eds.). Monteverde: Ecology and conservation of a tropical cloud forest. Oxford, New York. [ Links ]
Setzer, W.N., M.C. Setzer, A.L. Hopper, D.M. Moriarity, G.K. Lehrman, K.L. Niekamp, S.M. Morcomb, R.B. Bates, K.J. McClure, C.C. Stessman & W.A. Haber. 1998. The cytotoxic activity of a Salacia liana species from Monteverde, Costa Rica, is due to a high concentration of tingenone. Planta Med. 64: 583. [ Links ]
Setzer, W.N., M.C. Setzer, D.M. Moriarity, R.B. Bates & W.A. Haber. 1999. Biological activity of the essential oil of Myrcianthes sp. nov. "black fruit" from Monteverde, Costa Rica. Planta Med. 65: 468-469. [ Links ]
Setzer, W.N., M.C. Setzer, J.M. Schmidt, D.M. Moriarity, B. Vogler, S. Reeb, A.M. Holmes & W.A. Haber. 2000b. Cytotoxic components from the bark of Stauranthus perforatus from Monteverde, Costa Rica. Planta Med. 66: 493-494. [ Links ]
Setzer, W.N., M.N. Flair, K.G. Byler, J. Huang, M.A. Thompson, A.F. Setzer, D.M. Moriarity, R.O. Lawton & D.B. Windham-Carswell. 1992. Antimicrobial and cytotoxic activity of crude extracts of Araliaceae from Monteverde, Costa Rica. Brenesia 38: 123-130. [ Links ]
Setzer, W.N., M.T. Holland, C.A. Bozeman, G.F. Rozmus, M.C. Setzer, D.M. Moriarity, S. Reeb, B. Vogler, R.B. Bates & W.A. Haber. 2001. Isolation and frontier molecular orbital investigation of bioactive quinone-methide triterpenoids from the bark of Salacia petenensis. Planta Med. 67: 65-69. [ Links ]
Setzer, W.N., X. Shen, R.B. Bates, J.R. Burns, K.J. McClure, P. Zhang, D.M. Moriarity & R.O. Lawton. 2000a. A phytochemical investigation of Alchornea latifolia from Monteverde, Costa Rica. Fitoterapia 71: 195-198. [ Links ]
Sheets, P.D. & B.R. McKee. 1994. Archeology, volcanism, and remote sensing in the Arenal region, Costa Rica. University of Texas, Austin. [ Links ]
Timm, R.M. 2000. Prehistoric cultures and inhabitants, pp. 408-409. In N.M. Nadkarni & N.T. Wheelwright (eds.). Monteverde: Ecology and conservation of a tropical cloud forest. Oxford, New York. [ Links ]