Revista de Biología Tropical
versión On-line ISSN 0034-7744versión impresa ISSN 0034-7744
Rev. biol. trop vol.51 no.2 San José jun. 2003
Oryza latifolia (Poaceae) in Costa Rica
Ethel Sánchez 1, Mayra Montiel 2 & Ana M. Espinoza 3, 4
Abstract
The wild rice species Oryza latifolia is endemic to Tropical America, allotetraploid and has a CCDD genome type. It belongs to the officinalis group of the genus Oryza. This species is widely distributed through-out the lowlands of Costa Rica and it is found on different life zones, having great morphologic diversity. The purpose of this research is to perform a morphologic description of O. latifolia samples of three Costa Rican localities (Carara, Liberia and Cañas) and to see if the phenotypic diversity of the species is reflected at the ultra-structure level. Structures such as the leaf blade, ligule, auricles and spikelet were analyzed. Leaf blade morphology of the specimens from the three localities is characterized by the presence of diamond-shaped stomata with papillae, zipper-like rows of silica cells; a variety of evenly distributed epicuticular wax papillae and bulky prickle trichomes. The central vein of the leaf blade from the Cañas populations is glabrous, while those from Carara and Liberia have abundant papillae. There are also differences among the borders of the leaf blade between these locations. Cañas and Liberia present alternating large and small prickle trichomes ca. 81 and 150 µm, while Carara exhibits even sized prickle trichomes of ca. 93 µm. Auricles from Cañas are rectangular and present long trichomes along the surface ca. 1.5 mm, while those of Liberia and Carara wrap the culm and exhibit trichomes only in the borders. The ligule from the plants of Carara has an acute distal tip, while that of Cañas and Liberia is blunt. The Liberia spikelet has large lignified spines while Cañas and Carara show flexible trichomes.
Key words: Rice, Oryza latifolia, ultrastructure, morphology, anatomy, diversity.
Four wild species of the genus Oryza native Desv. of Central and South America and the Caribbean have been described: O. latifolia, O. alta, O. grandiglumis and O. glumaepatula. The first three are tetraploid (CCDD) and the latter is diploid (AA) (Vaughan 1994). In Costa Rica, all species are present except O. alta (Zamora et al. 1998, Zamora 2001).
Some of these wild relatives are resistant to diseases and pests that affect cultivated rice (Vaughan 1989, 1994), therefore, they represent useful sources of genes for the improvement of cultivated rice (O. sativa). The incorporation of these genes through interspecific crosses could improve yield, confer resistance to diseases and pests and would permit rice cultivation in areas currently inadequate for its production.
O. latifolia is distributed from Mexico to Brazil and the Caribbean Islands (Tateoka 1962a, 1962b). It is found in clear patches in the forests, in secondary forests, savannas, grasslands, cultivated fields, muddy soils, along the shores of rivers, lagoons and creeks (Pohl 1978, 1980). It is commonly known by the name of "arroz pato" and it flowers throughout the year (Zamora et al. 1998). In Costa Rica it is found in the lowlands, occupying diverse life zones, from the tropical dry forest (1200 mm annual precipitation) to the humid tropical forest (4000 mm annual precipitation) and in altitudes ranging between 0 m and 650 m above sea level (Zamora et al. 1998). The largest populations is found at Palo Verde National Park, Tempisque Conservation Area, Guanacaste, along the Tempisque River, in areas subjected to periodic floods. It is also very abundant in the Atlantic slopes, in the Tortuguero National Park, at the Tortuguero Conservation Area, Limón (Zamora et al. 1998).
This species presents morphologic and genetic variability, detected in the plant size (X: 178 ± 64.2 cms SD), spikelet size (ranging from 4.8 to 7.2 mm) awn length (X: 18 ± 18 mm SD), node color and size and number of branches in the panicle, among others (Zamora et al. 1999 a and b, Zamora 2001). The ligule is a stiff membrane (X: 3.29 ± 1mm SD), blunt, rigid and pubescent. The spikelet is composed of two sterile lemmas (glumes) and two fertile lemmas. The lemma, palea and floret include an oblong ovary with two feathery stigmas and six anthers composed of a filament and four lobes (Pohl 1978, 1980). Variability in isozyme patterns among Costa Rican populations have also been documented (Quesada et al. 1999, Quesada 2001).
The purpose of this work is to describe the ultrastructure of the leaf blade, ligule, auricles and caryopses from three populations of O. latifolia from three Costa Rican locations: Carara, Liberia and Cañas, to determine if intraspecific ultrastructural variability can be found.
Materials and methods
Material sampling: O. latifolia plants were collected at the Palo Verde National Park, Cañas, Guanacaste (accession CIBCM-122); at the Carara Biological Reserve, Orotina, Alajuela (accession CIBCM-022) and at Liberia, Guanacaste (accession CIBCM-004). Seeds of the collected samples were grown in the greenhouse at the Centro de Investigación en Biología Celular y Molecular, San Pedro de Montes de Oca, San José, Costa Rica.
Electron microscopy: Samples of the leaf, auricles, ligule and inflorescences were fixed in a 2.5% glutaraldehyde and 2% paraformaldehyde solution in a 0.1 M sodium phosphate buffer, pH 7.4 (Karnovsky 1965) and were rinsed with the buffer solution. The samples were post-fixed with 1% osmium tetraoxide in the sodium phosphate buffer, were rinsed with distilled water and dehydrated in a ethanol gradient. Four washes were performed with terbutylic alcohol and were later dried by sublimation (Sublimate Eiko ID-2, Japan). The samples were placed on aluminum bases and were covered with 20nm platinum (Ionic Cover Eiko IB-5, Japan). The samples were then observed in two Scanning Electron Microscopes (Hitachi S-570 y S-2360N), with an acceleration voltage of 15 KV. Anthers were homogenized with a 10% sodium hydroxide solution, rinsed with distilled water and then centrifuged at 2000 rpm (Hitachi 05 PR-22, Japan). They were later cleaned by ultrasound (Astrason 11H, Japan), fixed with 1% osmium tetraoxide in 0.1M sodium phosphate buffer and processed as previously described. The caryopses were placed in an incubator at 37ºC for 48 hours. They were then mounted, covered, and observed on Hitachi S-570 and S-2360N electron microscopes, with an acceleration voltage of 15 KV.
Results
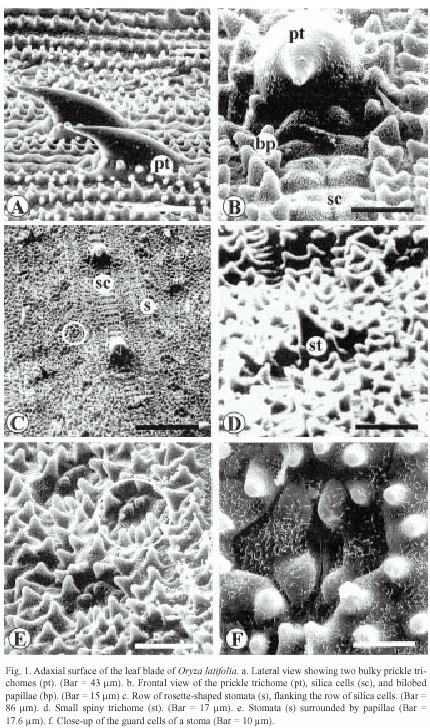
The ultrastructure of the adaxial surface of the Oryza latifolia leaf blade has very well-defined parallel vascular bundles, with a central and parallel to this are the lateral bundles. In the intercostal areas, long cells covered with abundant papillae and zipper-like silica cells were observed (Fig. 1b and 1c). These alternate with bulky prickle trichomes that measure between ca. 40 µm and ca. 63 µm (Figs. 1a and 1c). In addition, small trichomes of ca. 15 µm in length could be observed (Fig. 1c and 1d). The epicuticular wax pattern is rodlet-shaped (Fig.1f), and stomata are distinguished as rosettes at low magnification (Fig. 1e) and measure about 17 µm, and the subsidiary cells present various papillae (Fig. 1f).
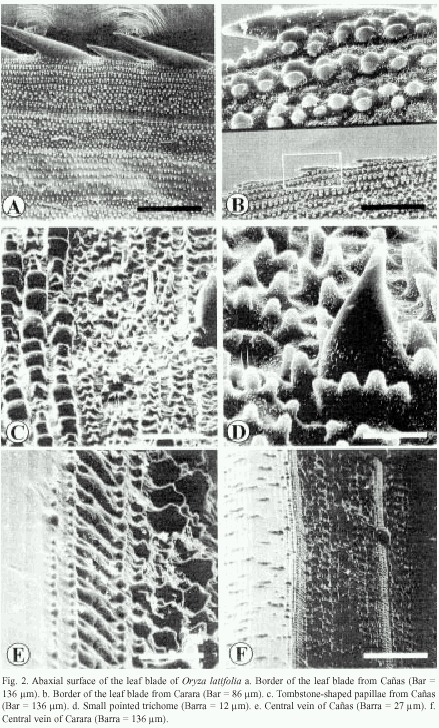
The borders of the leaf blade show a row of prickle trichomes (Fig. 2a and 2b). Those of Cañas and Liberia samples measure 65 to 81 and 120 to150 µm in length and are arranged alternatively (Fig. 2a), while in Carara, they are an even in size and measure about 93 µm (Fig. 2b). From the border inward, the epidermal pattern is as follows: several pairs of rows of wax papillae organized in groups, a row of stomata surrounded by papillae followed by another row of papillae and a row of silica cells. This pattern is repeated towards the interior of the leaf blade (Fig. 2a).
In the abaxial surface of the leaf blade, the following epidermal pattern can be observed: rows of stomata, trichomes, papillae and silica cells. This pattern is redundant along the blade surface and it is very similar to that of the adaxial surface. It is composed of papillae that measure about 6 by 9 µm, and in some cases, rows or groups of three to eight tombstone-shaped papillae can be observed. These papillae measure about 12 by 9 µm (Fig. 2c). The central surface of the central vein is composed of cells lacking epicuticular wax and papillae (Fig. 2e). The borders of the central vein have papillae, a row of silica cells and another set of papillae that increase height as they are located further away from the vein (Fig. 2e). Carara and Liberia have a central vein decorated with few small papillae that are distributed irregularly (Fig. 2f).
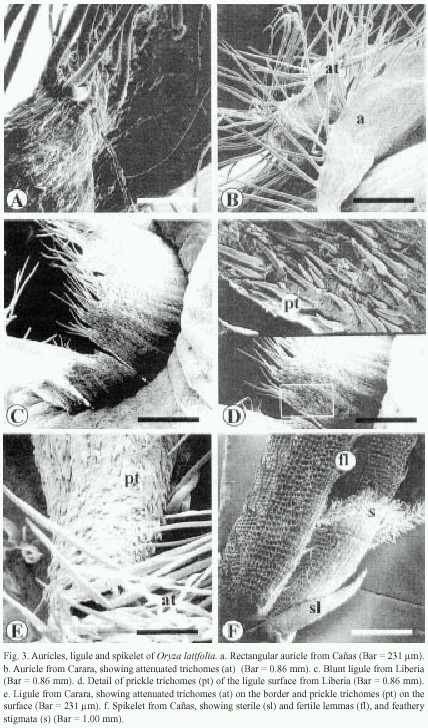
The auricles of Cañas are rectangular and their surface contain small, pointed trichomes and long attenuated trichomes. The latter are more abundant at the edges of the auricles (Fig. 3a). The auricles of Carara and Liberia wrap the culm, with long trichomes along the edges and small prickle trichomes on the surface (Fig. 3b).
The ligule of Cañas and Liberia is a stiff blunted membrane with prominent lateral vascular tissue, with abundant attenuated trichomes on its distal end (Fig. 3c), while at its surface, small and flexible trichomes are observed (Fig. 3d). The ligule from the Carara samples is, on the other hand, cone-shaped, pubescent and its distal tip is sharp with abundant attenuated trichomes (Fig. 3c). Additionally, it has short prickle trichomes that measure ca. 47 µm in length on its surface (Fig. 3e).
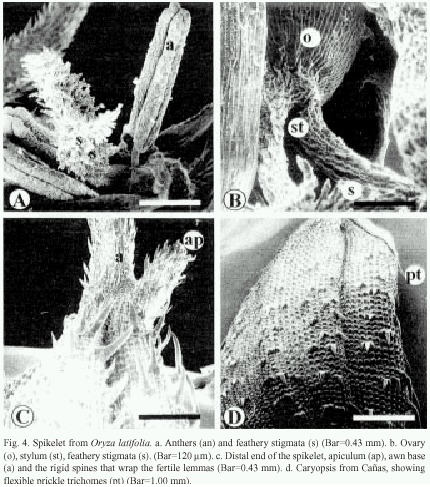
The sterile lemmas of the spikelet are arrow-shaped with serrated edges (Fig. 3f). The fertile lemmas are covered with rigid spines in Carara and Liberia samples (Fig. 3f, 4c), while those from Cañas exhibit flexible trichomes (Fig. 4d). The awn is covered by spiny trichomes and lignified papillae (Fig. 4c). The anthers have four lobes covered with long reticulate cells and abundant wax (Fig. 4a), and on tips have scaly surface. The feathery stigmata of the ovary are covered with short, irregular cells (Fig. 4a and 4b).
Discussion
After ultrastructural analysis of the three Oryza latifolia populations, intra-specific variability in the leaf blade was observed in relation to the ornamentation of the central vein, the trichomes of the borders of the leaf blade, and in the shapes of the auricles and ligule. This diversity was previously observed macro-scopically by Zamora and co-workers (1998, 1999 a, b and c) and Zamora (2001). In addition, molecular tests using isoenzimes also show those modifications (Quesada et al. 1999, 2001). Considering that the variability occurs in all evolutionary and speciation process, Samuel (1988) claim that it is possible that a gradual divergence on a population system may occur, as well as in a primary homogeneous species in two or more different population systems. This divergence is generally related to the adaptation to different geographical areas, climates, or well- defined ecological habitats, resulting in a genetic differentiation that is expressed both in the morphology and in the physiology of the species. As a result, it is possible to find divergent variations in the population depending on the environmental gradient at which the species is kept (Samuel 1988, Zamora 2001). The present analysis was done in the populations of O. latifolia from the South-Pacific zone and North zone of Costa Rica, so it is advisable to continue the analysis of the populations from other areas in order to determine the present variability in the species.
In relation to the other structures found in the leaf blade of O. latifolia, it was found that they are similar to the descriptions of Metcalfe (1960) and Hoagland and Paul (1978) for the botanical species Oryza and the morphology of the ligule, auricles and spikelet are in agreement with the descriptions done by Stand (1973), Pohl (1978), Hoagland and Paul (1978), Montiel and Kozuka (1994), Terrel, Peterson and Wergin (2001).
Acknowledgements
The authors want to acknowledge Enrique Freer for value collaboration, to Tania Quesada and Ricardo Jiménez for the translation of the manuscript to English, to Alejandro Zamora for valuable discussions, to Jéssica Coto for the art work.
Resumen
La especie silvestre Oryza latifolia es endémica de América, tetraploide y de genoma CCDD. Pertenece a las especies del género Oryza del grupo officinalis. Presenta una amplia distribución en las tierras bajas de Costa Rica y se le encuentra en varias zonas de vida, mostrando una gran diversidad morfológica. El propósito de este trabajo fue realizar una descripción ultraestructural de plantas de O. latifolia provenientes de las localidades Carara, Liberia y Cañas con el fin de determinar si hay variación intrapo-blacional. Se analizó la lámina foliar, lígula, aurículas y espiguilla, con énfasis en las estructuras de valor taxonómico. La morfología de la lámina foliar de los especímenes de las tres localidades se caracteriza por la presencia de estomas romboidales con papilas, células de sílice de forma crenada, variedad de papilas de cera, distribuidas en forma regular y tricomas espinosos abultados de ca 53 µm. La vena central de la lámina foliar de O. latifolia, localidad Cañas, es lisa, mientras que en Carara y Liberia presenta papilas. Los bordes de la lámina foliar difieren entre localidades; Cañas y Liberia presentan tricomas ganchudos grandes de ca. 150 µm y pequeños de ca 81 µm que alternan, mientras que Carara tiene tricomas ganchudos pequeños de igual tamaño (ca. 93 µm). Las aurículas de Cañas son rectangulares y presentan tricomas atenuados largos de ca. 1.5mm en toda la superficie, mientras las de Liberia y Carara son envolventes y presentan dichos tricomas solamente en los bordes. La lígula de Carara tiene el extremo distal agudo, mientras que Cañas y Liberia lo tienen truncado. La espiguilla de Liberia presenta tricomas espinosos lignificados grandes, mientras que Cañas y Carara tienen tricomas flexibles.
References
Hoagland, R.E. & R.N. Paul. 1978. Comparative SEM study of red rice and several commercial rice (Oryza sativa) varieties. Weed Science 26(6): 619-625. [ Links ]
Karnovsky, M.J. 1965. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J. Cell Biol. 27: 137A. [ Links ]
Metcalfe, C.R. 1960. Anatomy of the Monocotyledons I. Gramineae. Clarendon, Oxford. [ Links ]
Montiel, M.B. & Y. Kozuka. 1994. Los pólenes de gramíneas y su relación con alergias en el Neotrópico: el caso de Costa Rica. Rev. Biol. Trop. 42(Supl. 1): 1-39. [ Links ]
Pohl, W.R. 1978. How to know the grasses. 3th. edition. Dubuque, Iowa: Wm. C. Brown. [ Links ]
Pohl, W.R. 1980. Flora costaricensis, Gramineae. In W. Burger (ed.). Chicago, Field Museum of Natural History. [ Links ]
Quesada, T., J. Lobo & A.M. Espinoza. 1999. Diversidad genética de la especie silvestre de arroz Oryza latifolia y estudio del flujo genético entre poblaciones de diferentes regiones de Costa Rica. In Memorias, Jornadas de Investigación de la Universidad de Costa Rica, pp. 38. [ Links ]
Quesada,T. 2001. Diversidad genética de la especie silvestre de arroz Oryza latifolia Desv. en Costa Rica. Tesis de Maestría, Escuela de Biología, Universidad de Costa Rica, Costa Rica. [ Links ]
Samuel, B.J. 1988. Sistemática Vegetal. México, D. F. Fuentes. [ Links ]
Sánchez, E. 1999. Comparación morfológica ultraestructural de las especies silvestres de arroz (Oryza): Poaceae en Costa Rica. Tesis de Licenciatura, Escuela de Biología, Universidad de Costa Rica. Costa Rica. [ Links ]
Stand, M.Y. 1973. Scanning electron microscop silica bodies and other epidermal features in basis (Tradescantia) leaf. Bot. J. Linn. Soc. 233-244. [ Links ]
Tateoka, T. 1962a. Taxonomic studies of Oryza I, O. latifolia Complex. Bot. Mag. Tokyo 75: 418-427. [ Links ]
Tateoka, T. 1962b. Taxonomic studies of Oryza II. Several Species Complexes. Bot. Mag. Tokyo 75: 455-461. [ Links ]
Terrel, E.E., P.M. Peterson & W.P. Wergin. 2001. Epidermal Features and Spikelet Micromorphology in Oryza and Related Genera (Poaceae: Oryzeae). Washington, D. C. Smithsonian Contributions to Botany. Number 91. Smithsonian Institutions Press. [ Links ]
Vaughan, D.A. 1989. The genus Oryza L. Current status of taxonomy. IRPS. 138: 1-21., Manila, IRRI. [ Links ]
Vaughan, D.A. 1994. The wild relatives of rice. A genetic resources hand book. International Rice Research Institute (IRRI). Manila. [ Links ]
Zamora, A., E. Sánchez, T. Quesada, C. Muñoz, P. Porras, C. Quesada & A.M. Espinoza. 1998. Wild species, reservoirs of useful genes for the genetic improvement of rice. In International Symposium on Rice Germplasm Evaluation and Enhancement. Stuttgart, Arkansas, USA. 37 p. [ Links ]
Zamora, A., T. Quesada, P. González., J. Lobo & A.M. Espinoza. 1999a. Wild rice species of Costa Rica: diversity, distribution and potential use as source of genes for rice improvement. International Meeting of the Rice Biotechnology Program of the Rockefeller Foundation. Phuket, Thailand. 45 p. [ Links ]
Zamora, A., E. Sánchez, T. Quesada, P. González, C. Quesada, R. Gámez & A.M. Espinoza. 1999b. Especies silvestres, reserva de genes útiles para el mejoramiento del arroz. In Memorias, Jornadas de Investigación de la Universidad de Costa Rica. pp. 37. [ Links ]
Zamora, A., J. Lobo, & A.M. Espinoza. 1999c. Genética de poblaciones y biología reproductiva de la especie silvestre de arroz Oryza glumaepatula: conocimento básico para su uso como recurso genético. In Memorias. Jornadas de Investigación de la Universidad de Costa Rica, pp. 37. [ Links ]
Zamora, A. 2001. Diversidad morfológica y genética de las especies de Oryza (Poaceae) nativas de Costa Rica. Tesis de Maestría, Escuela de Biología, Universidad de Costa Rica, Costa Rica. [ Links ]
1. Centro de Investigación en Estructuras Microscópicas, Universidad de Costa Rica, Apdo 2060 San Pedro de Montes de Oca, San José, Costa Rica. Fax (506) 2073182, E-mail: ethels@cariari.ucr.ac.cr
2. Escuela de Zootecnia, Facultad de Agronomía, Universidad de Costa Rica, San José, Costa Rica.
3. Centro de Investigación en Biología Celular y Molecular, Universidad de Costa Rica, San José, Costa Rica. Fax (506) 207-3190, E-mail: amespino@racsa.co.cr .
4. Escuela de Fitotecnia, Facultad de Agronomía, Universidad de Costa Rica, San José, Costa Rica.












 uBio
uBio