Revista de Biología Tropical
versión On-line ISSN 0034-7744versión impresa ISSN 0034-7744
Rev. biol. trop vol.51 no.1 San José mar. 2003
Abstract
Drosophila gouveai is a cactophilic species endemic to South America. In southeast Brazil it is found on summits of isolated hills, which apparently are current refugia resulting from climatic changes during the Quaternary Period. It breeds only in necrotic cactus cladodes of Pilosocereus machrisii. Temporal differences in necrotic cactus availability could have a great impact upon D. gouveai population size, and could thus influence its evolutionary history. We analyzed the relationship between necrotic cactus availability and population size of D. gouveai. The fluctuation in the population size, variation in necrotic cactus availability and exploitation of this resouce by larvae were surveyed bimonthly for one year on a sandstone table hill in central-south Brazil. Temporal necrotic cactus availability did not vary significantly, though in June there was a moderate decrease. Larval populations were highest in October and December. The D. gouveai population size was highest in February and remained relatively stable the rest of the year. The observed fluctuation in population size was not a function of temporal necrotic cactus availability in quantitative terms.
Keywords: Cactus-yeast-Drosophila system, D. serido sibling set, Ecology, FPS, Patchy environment, Refugia, repleta group.
A common observation of population ecology is a fluctuation in the population size (FPS), often in response to the resource availability (Stephan and Wissel 1999; Dobzhansky and Pavan 1950). Such fluctuations in population size imply alterations in genetic variability within populations and in differentiation of populations. For instance, Frankham (1995) pointed out that FPS is the most important variable affecting the ratio of the effective to the total population size (Ne/N), a critical parameter for evolutionary genetics and wildlife management. Accentuated fluctuation in population size occurs in many Drosophila species in an annual cycle, generally because of the ecological specificity for breeding sites (Dobzhansky and Pavan 1950). Mangan (1982) and Breitmeyer and Markow (1998) found that cactophilic Drosophila species of the Sonoran Desert undergo substantial contractions in population size during the year, due to changes in rot availability. These authors inferred that availability of resources has a great influence on changes in genetic composition and on the evolution of the life history of Sonoran Desert species. This situation becomes even more critical in isolated populations with limited resources, such as the cactophilic species of South America.
The species Drosophila serido Vilela and Sene 1977 has an endemic geographical distribution in South America. It is found in areas of open vegetation (Vilela et al. 1980, 1983, Sene et al. 1982, 1988), and is always associated with cactus. It exclusively breeds on necrotic cactus cladodes, hereafter referred to as "rots", all year without interruption. In this cactus-yeast-Drosophila system, the larvae develop in rots by feeding on specific yeasts involved in this process (Pereira et al. 1983). Currently, cactus plants are patchily distributed in central-south Brazil causing isolation of this species (Vilela et al. 1980, 1983, Vilela 1983). Studies of the geographical distribution and of several markers (Sene et al. 1982, 1988, Silva and Sene 1991) showed that D. serido is a polytypic species composed of a group of closely related species. At the present time, this group is split into six species, including Drosophila gouveai (Tidon-Sklorz and Sene 2001). The D. gouveai populations are distributed in a xerophitic vegetation zone, from the "Caatinga" in northeast Brazil to the "Cerrado" (savanna) region in the middle west and southeast and the "Pantanal" ecosystem in the southwest. In Minas Gerais and São Paulo states, the populations of D. gouveai are found on the summits of isolated hills associated with the cactus Pilosocereus machrisii Dawson 1957 constituting a continental island situation.
Insular continental habitats have great importance due to their creative role of diversity, observed as a high endemism rate (see Prance 1996). These habitats can be formed in several ways. One example is the residual hills of sandstone in São Paulo state, named sandstone table hills, which shelter xerophitic vegetation on the hill tops in a very restricted area. They are compared in ecological and geomorphological terms to the "inselbergs" and "tepuis" of Amazonia studied by Prance (1996).
Fluctuation in population size might be one of the factors that promotes the differentiation found in the species of the D. serido sibling set, since these species have a high host specificity and live in areas with restricted resources. In order to determine the relationship between rot availability and population size of D. gouveai, bimonthly surveys were conducted of the rot availability in a sandstone table hill for one year. Fluctuations in population size and variation in the exploitation of the rots were also determined. These data were used to examine the hypothesis that rot availability influences the population size of cactophilic D. gouveai by testing the predictions that rot availability shows temporal variation in terms of their incidence at different times and that population size is associated with rot availability, such that the largest population is found at the time of most abundance of the resource. This work is a part of a study of isolation and host specificity effects on D. gouveai populations.
Material and methods
Study area: The study area is located in southeast Brazil where "Cerrado" vegetation (savanna) is predominant. This area is a sandstone table hill, called "Morro do Forno", located at 21º0216 S, 47º1918 W (Altinópolis, São Paulo State) and approximately 200 m elevation. The top has an area of approximately 6ha. Such hills are forms of residue relief, a result of lingering erosive processes, the tops are at the altitude of the relief surface that existed in the past (Ross and Moroz 1997). The tops of these hills are welldrained and become very dry in the dry season, sheltering a relict vegetation (rock field), called "campo rupestre" vegetation, adapted to xeric conditions. Some characteristic plants in these areas are the cactus P. machrisii, the Veloziaceae Xerophyta cinerascens Mart. Ex Schult. f. and Barbacenia fragrans Goeth and Henrand, the Bromeliaceae Pitcairnia haminea var. flocosa L.B. Smith and the Xiridaceae Xyris seubertii Alb. Nilsson., all with adaptations to drought (Giulietti et al. 1987). The climate of the region is tropical being characterized by warm temperatures and by an irregular distribution of rainfall during the year. The temperature varies according to the latitude and elevation. The mean annual temperature of this hill is 22ºC. During the warmest period (September to March) the mean monthly temperature is 24ºC and January is usually the warmest month. In the coldest period (May to August) the temperature is always below 18ºC during at least one month; the coldest month is June or July (Nimer 1989). The rainfall is concentrated in the warmest months (December to February) when 50% of the precipitation occurs. The relatively cold months are dry (Salgado-Labouriau et al . 1998).
Collection of adult flies: The collections were made bimonthly during one year, from August 1996 to June 1997. The flies were captured on orange and banana baits fermented with bakers yeast, placed in cans hung from trees about 1,5m above the ground, remaining in place for three days (Sene et al.1981). In each survey 15 bait traps were placed in the area infested with P. machrisii. All flies were brought alive to the laboratory and all individuals of the species D. gouveai were identified. The fluctuation in population size was studied by determining the number of D. gouveai flies captured in each trap in the survey. The capture of flies from other populations could have affected the results. The risk of this happening was considered minimal since the bait we used is only attractive over short distances (see Johnston and Heed 1975), and the closest population was about 10km away.
Host plant survey: In order to verified the resource availability a study was made of the temporal variation in cactus necrosis in terms of incidence at different times of the year.
P. machrisii is a relatively small-sized columnar cactus with cylindrical stems 1-2m long and 8-12cm in diameter. Each plant produces 5-10 stems on average, and necrosis occurs individually in each stem. Surveys of the availability of rots were made about every two months for eight months, from October 1996 to June 1997. The four observations were made on three, approximately 150m2 , plots. The cacti on each plot were mapped and the number of stems on each plant was counted in all surveys. The variation in the number of stems on each plant was caused by the occurred necrosis, and with this counting the availability of this resource could be considered in each studied period. The stems were classified in two sizes so that the new stems that sprang up did not interfere in the counting. A necrosis rate (ratio of necrotic stems in relation to the total of sample stems in each period) was calculated.
Collection of larvae: Throughout all the surveys, the rots were picked up in the all study area, except for the June survey, when no rot was found. The necrotic stem cacti were weighed and placed in terraria. The emerging flies were identified and counted, and the rate of emerged flies was calculated as a ratio of the number of flies to stem weight. Alterations in this rate could indicate a change in the degree of exploitation of that resource. The cactus P. machrisii is the only breeding resource for D. gouveai in the Morro do Forno locality.
Results
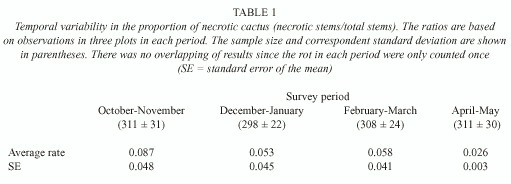
Resource availability: Temporal variation in cactus necrosis was determined (Table 1). The April-May period had the smallest average rate of necrosis, while the October-November period had the largest rate. A one-way ANOVA indicated that the differences among these periods were not significant (F3,8 = 0.45; P= 0.724).
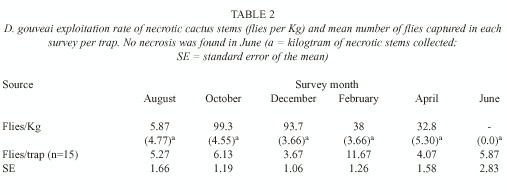
Necrosis exploitation: The rate of rot exploitation was calculated as the number of flies emerged in each kilogram of necrotic stem (Table 2). On average, each stem weighed 1.3kg. This does not include only necrotic tissues, but also tissues which still have not suffered necrosis. The exploitation of the rots was much more intense in both October and December. The smallest exploitation rate was in August and intermediary rates were observed in February and April. The robustness of the results made any statistical test unnecessary.
No rots were found in the June sampling. This does not mean that this resource was not available at that time.
Fluctuation in the population size:
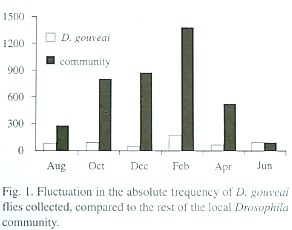
There was fluctuation in the population size during the year, but in no month did D. gouveai become rare among the local Drosophila community (Table 2). An ANOVA one-way for log-transformed absolute frequency of D. gouveai in each trap indicated a significant difference among the surveys (F5,84 = 2.89; P<0.02). Multiple comparisons by the Bonferroni procedure (Neter et. al. 1996) revealed that the collections of August, October, December, April and June had, on the average, the same frequency (5.00; SE=0.80), while February had a significantly larger frequency, estimated as 11.67 (SE=0.70).
Those results were supported when the absolute frequency of the total flies captured in each collection was analyzed through an equality proportion test. The proportions among the monthly surveys differed significantly (X25 =102.54; P<0.001). The results for December and February contributed the most to this value.
To determine if there was an association between the FPS in D. gouveai and the availability of rots, the correlation between these two variables was calculated. Due to the generation time of D. gouveai, a variation in the availability of rots would cause changes in the population size of the flies after 30–60 days. Therefore, the rate of necrosis was compared to the population size two months later (the numbers in tables 1 and 2 were used for this analysis). The test showed that there is no significant relationship between these two parameters, even though the r value is close to 1 (r =0.801; P > 0.05).
To determine if the fluctuations in the D. gouveai population size followed those of the local Drosophila community, a Chisquare homogeneity test was made to check the uniformity of proportions in both groups. This test showed that the proportions of D. gouveai among the other Drosophila species were heterogeneous among the monthly surveys (X25 =298.52; P<0.001). The population increase from December to February was similar for D. gouveai and the community. However, it is believed that the factors involved in the increase of the D. gouveai population size are different from those which influenced the expansion in the total community size. Otherwise, different directions in the results would not have occurred, such as in December, when the community size increased and D. gouveai declined. Figure 1 shows the relationship of fluctuation in population size of D. gouveai to that of the local Drosophila community.
Discussion
There was a high populational growth in D. gouveai in February and the population did not change significantly at any time of the year. FPS of this species did not follow the variations in the local Drosophila community, in spite of the similarity of fluctuations in some months. Morais et al. (1994) studying the feeding behavior of D. gouveai in the yeast communities of rots of Pilosocereus arrabidae showed that there is a niche partitioning in feeding between larvae and adult flies. They found that adult fly feeding is not limited to cactus yeast and they can be considered ecological generalists. Therefore the factors involved in the fluctuation of population size are specific to D. gouveai. Otherwise, the fluctuation in D. gouveai should have the same behavior observed in the Drosophila community on the Morro do Forno site.
The availability of rots, in terms of their incidence at different times of the year, was constant from October to April and it decreased approximately 50% between April and June (this difference was not significant). This quantitative variation in resource availability did not signficantly affect the size of the fly population. It is expected that an elevation or a fall in the availability of rots would cause alterations in the population size after a period of 30-60 days due to the generation time of this organism. Although this effect was not verified, it could be infered that another type of temporary variation in cactus necroses influenced the growth of the D. gouveai population in February. Rate of rot exploitation results indicates which variation this could be responsible for.
The rate of rot exploitation was great in both October and December, being 250% larger than in the other months. This increase could be due to a more favorable composition of the yeast community involved in the necrosis process in this period, which might make the substratum more suitable for the larvae. This yeast flora would benefit the development of the larvae, consequently elevating the emergence rate and causing in a following period an increase in population size.
In the Sonoran Desert, Breitmeyer and Markow (1998) found that the population size of four species of cactophilic Drosophila was related to abundance of resources in terms of patch size and duration of the necroses. However, in the summer the relationship between the resource availability (in terms of availability during different seasons) and the population size breaks down completely because the rot availability is high, but all flies species are rare. In addition, they also found that in the spring a decrease in the rot utilization occurred. The authors invoked some explanations to clarify this fact, among them the possibility of differences in the attractiveness of the necrosis in summer.
In fact, alterations in the yeast community composition in cactus necroses could be proven through the yeast community comparative study in different times of the year. Surveys of yeast species in colummar cacti rots in North America (Heed et al. 1976, Starmer et al. 1976, Starmer et al. 1982) and assays of yeast species from rotting Opuntia cactus in Australia (Barker et al. 1987) indicate a diversity of species among rots at different times within a locality (temporal heterogeneity). Barker et al. (1983) also found significant differences among seasons in the relative frequencies of the ten most common yeast species in rotting Opuntia stricta Haw in Australia. Furthermore, Vacek (1982) and Vacek et al. (1985) demonstrated substantial differences in nutritional sufficiency of these yeast species for larval development of Drosophila buzzatii, a species related to D. gouveai.
Therefore, the quality of the necrosis, in terms of the yeast community composition, should be the most influential variable on the population size of D. gouveai observed in our study. Thus, the observed FPS could occur as a function of the availability of necroses in terms of suitability of the substrate, caused by the yeast community composition. In October and December the necroses could be more suitable resulting in a greater rate of exploitation. This would lead to an increase in the population size which was found in February. After this, a return to original conditions would provoke a reduction in the rate of exploitation, resulting in the decrease in population that was found.
The use of the baiting technique in our work instead of other techniques such as mark-recapture, used by Breitmeyer and Markow (1998), is justified by the characteristics of the habitat and our research objectives. The great variety of microhabitats and feeding sites results in a wide distribution of the population in the study area. There is no site with a great concentration of flies of a single species, different from the case of the Sonoran Desert where a single cactus and a single Drosophila species predominate. This hinders a direct assessment of the population size in feeding and breeding places, as would be the case in the mark-recapture technique, making the baiting technique more appropriate.
The fluctuation in the population size observed in D. gouveai on Morro do Forno site might play a significant role in its evolutionary history. Due to their ecological specificity these populations, as well as other species of the D. serido sibling set, accompanied the successive expansions and retractions of the xeric vegetation in South America caused by paleoclimatic cycles during the last 36000 years (Van der Hammen 1974, AbSaber 1977a,1977b, Prance 1982). As a further feature, this species habitat was broken into fragments in the central-south area of Brazil, since the cactus Pilosocereus machrisii only occurs at the summits of hills amid a remnant rock field vegetation ("campo rupestre"). This constitutes isolated populations in an continental island situation.
Monteiro and Sene (1995) found that on each hill the D. gouveai populations can be distinguished through morphometric analysis of the aedeagus (male genitalia), as there is significant morphologic differentiation among the populations of the studied hills. The sandstone table hills can offer conditions to facilitate fast differentiation of D. gouveai populations, as has been found for several plant species located in "Inselbergs" and "Tepuis" of Amazonia, that show a high endemism (Prance 1996). We consider these sandstone table hills as current refugia, in similar terms to those tendered by Haffer (1969) and by Vanzolini and Williams (1970). This situation, followed by FPS, would contribute to differentiation among these populations. The fluctuation in the demographic parameters could change the amount of genetic differentiation among populations by affecting parameters, such as heterozygosity and F ST , Wrights measure of genetic differentiation among populations (Whitlock 1992, Moraes and Sene 2002).
The results of this work throw light on the role of FPS in the differentiation observed in the D. gouveai and in other species of the D. serido sibling set. Finally, only studies on the temporal fluctuations in genetic variability can more safely state the importance of this parameter in the process of speciation of this group.
Acknowledgments
We are grateful to C.A. Perez and his students from EPM-Universidade Federal de São Paulo for help with statistics, to D. De Jong for kindly reading the manuscript and to P.R. Epifânio for technical assistance. We are thankful also to S.P. Ulquiza and D. Gavio for help with the Spanish language and to three anonymous reviewers for their helpful comments. This research was supported by FAPE-SP grant number 96/5489-0, FINEP, CNPq and USP.
Resumen
Drosophila gouveai es una especie cactófila endémica de Sudamérica. En el sudeste de Brasil ella es encontrada en la cima de colinas aisladas, las cuales son actualmente refugios ambientales resultantes de los cambios climáticos acaecidos durante el Período Cuaternario. Ellas crían sólo en cladodios necróticos del cactus Pilosocereus machrisi i. Diferencias temporales en la disponibilidad de estos cladodios necróticos podrían tener un gran impacto sobre los tamaños de las poblaciones de D. gouveai, y por tanto influenciar su historia evolutiva. Nosotros analisamos las relaciones entre la disponibilidad de cactus en putrefacción y el tamaño de las poblaciones de D. gouveai. La fluctuación en los tamanõs poblacionales, la variación en disponibilidad de cactus y la explotacion deste recurso por larvas fueron analizadas bimensualmente y durante un año en un pico aislado de arenito, en el Centro-Sudeste de Brasil. La disponibilidad de cactus en putrefación no varió significativamente en el tiempo, excepto durante junio, cuando se registró una caída de aquella. Las poblaciones de larvas fueron mayores en Octube y Diciembre. El tamaño poblacional de D. gouveai fue mayor en Febrero, permaneciendo relativamente estable el resto del año. La fluctuación observada en la población no se comportó como función, en términos cuantitativos, de la disponibilidad de cactus en putrefacción.
References
AbSaber, A.N. 1977a. Espaços ocupados pela expansão dos climas secos da América do Sul, por ocasião dos períodos glaciais quaternários. Paleoclimas 3: 1-19. [ Links ]
AbSaber, A.N. 1977b. Os domínios morfoclimáticos da América do Sul. Geomorfologia 52: 1-23. [ Links ]
Barker, J.S.F., G.L. Toll & P.D. East. 1983. Heterogeneity of the yeast flora in the breeding sites of cactophilic Drosophila. Can. J. Microbiol. 29: 6-14. [ Links ]
Barker, J.S.F., W.T. Starmer & D.C. Vacek. 1987. Analysis of spatial and temporal variation in the community structure of yeast associated with decaying Opuntia cactus. Microbial Ecology 14 (3): 267-276.
Breitmeyer, C.M. & T.A. Markow. 1998. Resource availability and population size in cactophilic Drosophil a. Func. Ecol. 12: 14-21. [ Links ]
Dawson, Y. 1957 The machris brazilian expedition. Botany: a new columnar cactus from Goiás. Los Angeles Country Museum Contr. Science 10: 1-8. [ Links ]
Dobzhansky, Th. & C. Pavan. 1950. Local and seasonal variation in relative frequencies of species of Drosophila in Brazil. J. Anim. Ecol. 19: 1-14. [ Links ]
Frankham, R. 1995. Effective population size/adult population size ratios in wildlife: a review. Genet. Res. 66: 95-107. [ Links ]
Giulietti, A.M., N.L. Menezes, J.R. Pirani, M. Meguno & N.G. Wanderley. 1987. Flora da Serra do Cipó, Minas Gerais: caracterização e lista das espécies. Boletim de Botânica da Univers. de São Paulo 9: 1-151.
Haffer, J. 1969. Speciation in Amazonian forest birds. Science 165: 89-98. [ Links ]
Heed, W.B., W.T. Starmer, M. Miranda & M.W. Miller. 1976. An analysis of the yeast flora associated with cactophilic Drosophila and their host plants in the Sonoran Desert and its relation to temperature and tropical associations. Ecology 57: 151-160.
Johnston, J.S., W.B. Heed. 1975. Dispersal of Drosophila: the effect of baiting on the behavior and distribuition of natural populations. Am. Nat. 109: 207-216. [ Links ]
Mangan, R.L. 1982. Adaptation to competition in cactus breeding Drosophila. p. 257-272. In J.S.F. Barker & W.T. Starmer (eds.).Ecological Genetics and Evolution: the Cactus-Yeast-Drosophila Model. System Academic, Sydney. [ Links ]
McCauley, D.E. 1993. Genetic consequences of extinction and recolonization in fragmented habitats. p. 217-233. In P.M. Kareiva, J.G. Kingsolver & R.B. Huey (eds.). Biotic Interactions and Global Change. Sinauer Associates, New York. [ Links ]
Monteiro, S.G. & F.M. Sene. 1995. Estudo morfométrico de populações de Drosophila serido das regiões Central e Sul do Brasil. Rev. Bras. Genet. 18 (Suppl.): 283.
Moraes, E.M. & F.M. Sene. 2002. Breeding structure of an isolated cactophilic Drosophila population on a sandstone table hill. J. Zool. Syst. Evol. Research. 40: 123-128.
Morais, P.B., C.A. Rosa., A.N. Hagler & L.C. Mendonca-Hagler. 1994. Yeast communities of the cactus Pilosocereus arrabidae as resource for larval and adult stages of Drosophila serid o. Antonie van Leeuwenhoek 66: 313-317. [ Links ]
Neter, J., M.H. Kutner, C.J. Nachtsheim & W. Wasserman. 1996. Applied Linear Statistical Models. Times Mirror Higher Education Group. USA.
Nimer, E. 1989. Climatologia do Brasil. IBGE Publications, Rio de Janeiro. Pereira, M.A.Q.R., C.R. Vilela & F.M. Sene. 1983. Notes on breeding and feeding sites of some species of the repleta group of the genus Drosophila (Diptera, Drosophilidae). Ciênc. Cult. 9: 1313-1319.
Prance, G.T. 1982. Biological Diversification in the Tropics. Columbia University Press, New York. [ Links ]
Prance, G.T. 1996. Islands in Amazonia. Phil. Trans. R. Soc. Lond. 351: 823-833.
Ross, J.L.S. & I.C. Moroz. 1997. Mapa geomorfológico do Estado de São Paulo. FFLCH-Universidade de São Paulo, Instituto de Pesquisas Tecnológicas and Fundação de Amparo à Pesquisa do Estado de São Paulo-Mapas e Relatórios. São Paulo.
Salgado-Labouriau, M.L., M. Barberi. K.R. Ferraz-Vicentini & M.G. Parizzi. 1998. A dry climatic event during the late Quaternary of tropical Brazil. Rev. Palaeobot. Palynol. 99: 115-129. [ Links ]
Sene, F.M., M.A.Q.R. Pereira, C.R. Vilela & N.M.V. Bizzo. 1981. Influence of different ways to set baits for collection of Drosophila flies in three natural environments. Drosophila Inf. Serv. 56: 118-121.
Sene, F.M., M.A.Q.R. Pereira & C.R. Vilela. 1982. Evolutionary aspects of cactus breeding Drosophila species in South America. p. 97-106. In J.S.F. Barker & W.T. Starmer (eds.). Ecological Genetics and Evolution: the Cactus-Yeast-Drosophila Model System . Academic Press, Sydney.
Sene, F.M., M.A.Q.R. Pereira & C.R. Vilela. 1988. Contrasting patterns of differentiation inferred from traditional genetic markers in the process of speciation. Pacif. Sci. 42: 81-88. [ Links ]
Silva, A.F.G. & F.M. Sene. 1991. Morphological geographic variability in Drosophila serido (Diptera, Drosophilidae). Rev. Bras. Entomol. 35: 455-468.
Starmer, W.T., W.B. Heed, M. Miranda, M.W. Miller & H.J. Phaff. 1976. The ecology of yeast flora associated with cactophilic Drosophila and their host plants in the Sonoram Desert. Microb. Ecol. 3: 11-30.
Starmer, W.T., H.J. Phaff, W.B. Heed, M. Miranda & M.W. Miller. 1982. The yeast flora associated with the decaying stems of columnar cacti and Drosophila in North America. Evol. Biol. 14: 269-295. [ Links ]
Stephen, T., C. Wissel. 1999. The extinction risk of a population exploiting a resource. Ecol. Modell. 115: 217-225. [ Links ]
Tidon-Sklorz, R., F.M. Sene. 2001. Two new species of the Drosophila serido sibling set (DIPTERA, Drosophili-dae). Iheringia Série Zoologia 90: 141-146. [ Links ]
Vacek, D.C. 1982. Interactions between microorganisms and cactophilic Drosophila in Australia. p. 175-190. In J.S.F. Barker & W.T. Starmer (eds.). Ecological Genetics and Evolution: the Cactus-Yeast-Drosophila Model System. Academic, Sydney.
Vacek, D.C., P.D. East, J.S.F. Barker & M.H. Soliman. 1985. Feeding and oviposition preferences of Drosophila buzzatii for microbial species isolated from its natural environment. Biol. J. Linn. Soc. 24: 175-187. [ Links ]
van der Hammen, T. 1974. The pleistocene changes of vegetation and climate in tropical South America. J. Biogeogr. 1: 3-26. [ Links ]
Vanzolini, P.E. & E.E. William. 1970. South american anoles: the geographic differentiation and evolution of the Anolis chrysolepsis species group (Sauria, Iguanidae). Arquivos Zool. 19: 1-298. [ Links ]
Vilela, C.R. 1983. A revision of the Drosophila repleta species group (Diptera, Drosophilidae). Rev. Bras. Entomol. 27: 1-114. [ Links ]
Vilela, C.R. & F.M.Sene. 1977. Two new neotropical species of the "repleta group" of the genus Drosophila (Diptera, Drosophilidae). Papéis Avulços de Zoologia 30 (20): 295-299.
Vilela, C.R., F.M. Sene & M.A.Q.R. Pereira. 1980. On the Drosophila fauna of Chaco and East slopes of the Andes in Argentina. Rev. Bras. Biol. 40: 837-841. [ Links ]
Vilela, C.R., M.A.Q.R. Pereira & F.M. Sene. 1983. Preliminary data on the geographical distribuition of Drosophila species within morphoclimatic domains of Brazil. II. The repleta group. Ciênc. Cult. 35: 66-70.
Whitlock, M.C. 1992. Temporal fluctuations in demographic parameters and the genetic variance among populations. Evolution 46: 608-615.
1 Departamento de Genética, FMRP, Universidade de São Paulo. Ribeirão Preto, São Paulo. Brazil
* Corresponding authors adress: Departamento de Genética, FMRP, Universidade de São Paulo. Ribeirão Preto, São Paulo. 14049-900, Brasil. Fone: 55 16 6023103; Fax: 55 16 6336482; famesene@usp.br












 uBio
uBio 

