Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.50 n.3-4 San José Dec. 2002
In this study, the rates of tissue regeneration and recovery from injuries that emulated the bites of either butterfly or parrotfish on colonies of Montastraea annularis exposed to different sedimentation regimesp were determined. Two small reef patches were chosen elose to key Dos Mosquises, north of the Venezuelan mainland. Sixteen colonies (8 treatments + a single replicate) were artificially damaged at each patch and their recovery was monitored for three months by photographic means. The lesions were inflicted using two different techniques: scratching the polyps with a hard-nylon brush to resemble parrotfish (Scaridae) damages (Lesions Type 1) or jetting out the tissue with a syringe to simulate butterflyfish (Chaetondontidae) bites (Lesions Type 2). The diameter of the wounds ranged from 5 (small lesion) to 8 cm (large lesions) and both kinds were inflicted on the top and bottom of the colonies, with a single replicate for each treatment. The main factors affecting the recovery of the colonies' surface were lesion features (type, position and size), turbidity and chiefly, the sedimentation rate. WhiIe lesion recovery was slow where sedimentation and resuspension rates were high, tissue regeneration was improved under low sedimentation and resuspension conditions. Moreover, lesions located at the bottom of colonies regenerated completely, whereas sediments frequently covered top lesions and limited their recovery. More than 60% of the colonies with small lesions recovered almost completely in less than 90 days, whereas those with larger injuries frequently showed extensions of their damage and increased mortality. Tissue-only lesions (LT2) regenerated two to three times faster than those involving both tissue and skeletal damage (LT1).Other variables not controlled in this study, such as diseases, encrusting organisms overgrowth and herbivory introduced further variability to the regeneration rates.
Key words
Tissue regeneration, Montastraea annularis, sedimentation rates, recovery, artificial damages, Archipiélago de Los Roques, corals.
During the last two decades the trend of worldwide degradation of coral reefs has been related to habitat loss and overexploitation of their resources, as well as to natural disturbances such as hurricanes (Loya 1976 , Brown 1987 , 1990 ) and global warming (Harvell et al. 1999 ). The current decline of coral reefs has also been related to direct damages caused by anchors, grounding and mechanical extraction of reef organisms, all of which may particularly affect scleractinean corals. These damages has been classified into two main groups: (1) Tissue removal, and (2) Skeleton and tissue loss. The morphology of these colonial organisms allows for the partial loss of their modules whereas the polyps can survive and subsequently regenerate (Reusik 1997 ).
The study of lesion recovery in corals is a relatively new aspect of coral reefs science; most of the research done on this subject has been carried out in Curacao (Bak et al. 1977 , Bak and Van Es 1980 , Bak 1983 , Meesters and Bak 1993 , 1995 , Meesters et al. 1994 , 1996 , 1997 , Nagelkerken and Bak, 1998 ), Panama (Guzmán et al. 1994 ), Florida (Hayes and Bush 1990 ), the Red Sea (Oren et al. 1998 ) and Australia (Hall 1997 ). Only few studies that have focused on environmental factors as regulators of tissue regeneration processes have been performed. For instance, Lester and Bak (1985) studied the effects of environment in tissue regeneration on the reef-building coral Montastraea annularis (Ellis and Solander 1786). More recently Woesik (1998) compared the lesion healing capability in Porites lutea and P lobata corals from Japan, but he found no differences in tissue regeneration between these two species.
Like in many other Western Atlantic locations, M. annularis, Montastraea faveolata and Montastraea franksi are conspicuously predominant at Los Roques National Park coral reefs. Due to its phenotypic plasticity, high reproductive fitness and competition abilities, the first species is widely distributed and can be easily found in reef patches and forming both, fringing and barrier reefs, covering a wide range of depths (Foster 1979 , Weil and Knowlton 1994 , Van Veguel et al. 1996 , Knowlton et al. 1997 ). Even though the Los Roques coral reef complex is the largest and most important in Venezuela (Amend 1992 ), studies concerning coral injury recovery have not been conducted there before. In this paper we show how M. annularis responds to the disturbance produced by both tissue and skeletal removal. The progress of recovery of these lesions was also studied and comparisons were made between specimens from two reef patches with different sedimentation regimes.
Materials and methods
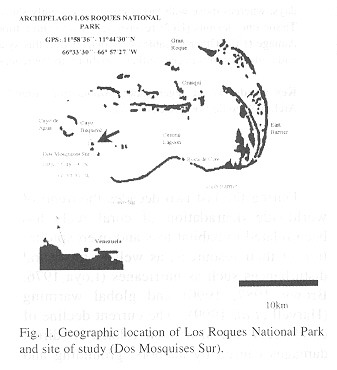
Study site: The present study was carried out at the Archipiélago Los Roques National Park, which is located 160 km north of the Venezuelan coast (11º 44' 45" to 11º 58' 36" N, 66º 32' 42" to 66º 52' 57" W). The Archipelago has more than fifteen coralina Keys and over two hundred banks. The keys form an irregular oval around a shallow lagoon, which is surrounded by to large barrier reefs; the eastern barrier is 20 km long, and the southern barrier is 30 km long (Fig. 1 ). The experiments were done in Dos Mosquises, a Key located on the South-western edge of the Archipelago (11º 48' N, 66º 53'W.) Dos Mosquises is protected by a horseshoe barrier reef, which is separated from the shoreline by a lagoon of shallow waters with a depth that rarely exceeds 4 m. The Key has a fringing reef 150 to 240 m wide and it has a maximal depth of 40 m (Hung 1985). Experiments were carried out between March and June of 1998, in two sites each under different sedimentation regimes but of similar depth (1-1.5 m). The first site, (S1) was on the horseshoe reef, 500 m off the shoreline; this site was subjected to low sedimentation rates. The second site, (S2) was on the fringing reef lagoon, close to the coastline, and with higher sedimentation rates.
Coral injuries: Sixteen healthy colonias (8 treatments + a single replicate per treatment) with no signs of injuries or bleaching were selected at each reef site; the chosen colonies ranged from 30 (small) to 60 cm 2 (large) and all were at the same depth (1-1.5 m). Three injury treatments were performed, according to size (small and large), type (skeletal and tissue loss) and position of the lesions (top and bottom of the colony). The diameter of small wounds ranged from 4.5 - 5 cm , while the larger ones ranged from 7.5 to 8 cm. Skeletal lesions (Type 1) were made by scratching the coral surface with a hard-nylon brush, while tissue loss (Type 2) was performed by jetting out the tissue from the polyps using a syringe. Type 1 lesions simulated the damage caused by parrotfish injuries (Bruckner and Bruckner 1998 ), while Type 2 lesions resembled to Chaetodontidae damages ( Ohman et al. 1998 ).
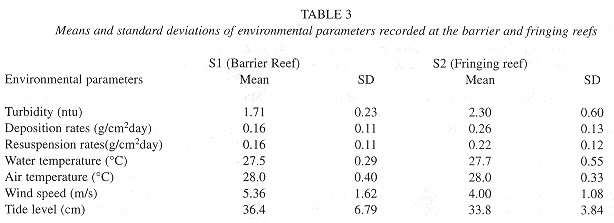
These lesions were monitored at three, five, eight, 17, 20, 60 and 90 days after the colonies were injured, using an underwater camera separated 10 cm from the damaged colonies. A frame adapted to the camera (Motor Marine 11) macro-lens was used in order to take all the photographs always at the same distance and at the same angle (90º). Daily records of air and surface water temperature, wind speed, turbidity and tide level were taken at each location. Turbidity was measured "in situ" using a digital turbidimeter, temperature was recorded with a hand thermometer and the wind speed with a digital anemometer. Six PVC sediment traps (15 cm high x 6cm diameter) and six Petri capsules (2 cm high x 6cm in diameter) were placed on concrete supports to estimate the sedimentation and resuspension rates, respectively. Each collector was changed every three days at cach reef site: the collected material was washed with freshwater, dried at 30 Cº for 48hr and then weighed.
All the areas undergoing recovery were calculated from pictures of the colonies by drawing them on transparent paper, clipping and weighing these shapes and comparing those weights with that of a known standard square of the same paper. The results were expressed in cm2 . The tissue regeneration rates (Ts) were obtained by calculatinc, the difference between the areas of the recovered surfaces (Ra) for any given interval (T1 and To in days), as follows:
Ts = [Ra (T 1 ) - Ra (To)] / (T1 - To)
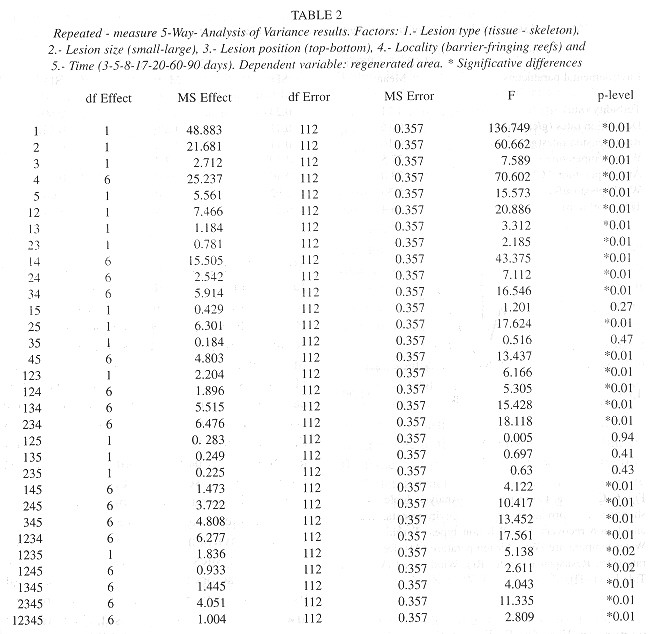
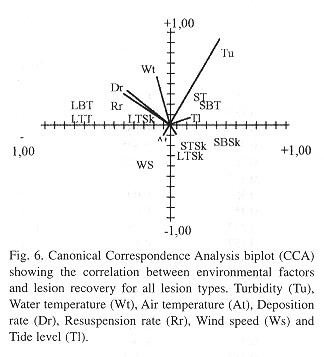
Statistical analysis: To test the effects of each treatment on regenerated areas between reef sites and monitoring days; we used a repeated-measures five-way analysis of variance (Zar 1998 ). The factors included in this analysis were: 1. Lesion type (tissue and skeleton), 2. Position (top-bottom), 3. Size (smalllarge), 4. Locality (barrier and fringing reef) and 5. Time (3, 5, 8, 17, 20, 60, 90). We also used a canonical correspondence analysis (CCA) (Jongman et al. 1995) with environmental variables measured at both reef sites, and the regenerated areas to determine the variables significantly associated to the recovery of lesions.
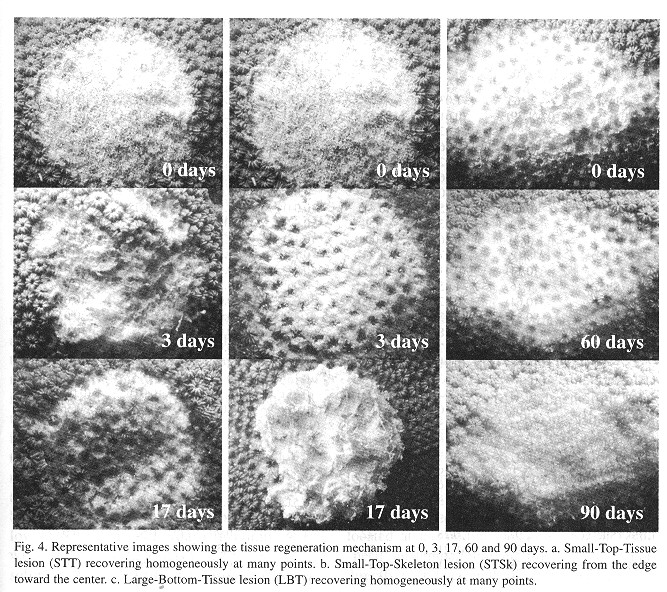
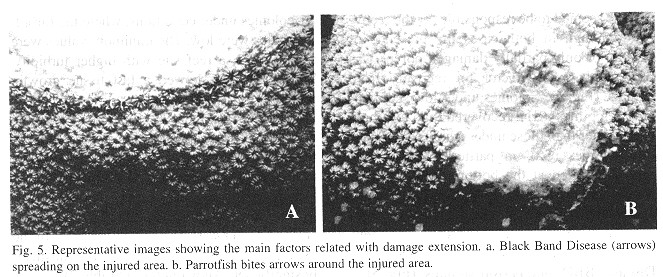
The species M. annularis recovered, rapidly from the artificially inflicted injuries, as it look just over twenty days for most of the colonies to regenerase the removed tissue. Injuries at the bottom position of the colonies recovered faster than those located on top of them, and Type 2 lesions (tissue) recovered faster than Type 1 lesions (skeleton and tissue). In addition, about 60% of the smaller injuries regenerated their tissue almost completely over the 90 days of monitoring ( Figs. 2 and 3 ). For all lesion Types, tissue recovery showed two stages: 1. A fast growing (1-2cm2/day) of a thin layer of new tissue during the first 20 days. 2. The regeneration of new polyps; which gradually regained their pigmentation. The total recovery of the injuries took 20 to 30 days in some cases, or from three to four months in others, depending on lesion features; and two different regeneration mechanisms were observed. In one, tissue growth started at many places over the injured surfaces, perhaps "activated" by healthy tissue inside those polyps that were injured but not destroyed. In the other case, tissue grew from the edges towards the center of the lesions. In this latter case healthy polyps surrounding the affected surface seemed to be responsable for the recovery, although in both cases the presence of healthy or only partially damaged polyps was presumably a key factor for recovery from damage ( Fig. 4 ). Colonies under low sedimentation regimes (SI) recovered two or three times faster than those under a high sedimentation regimes (S2), in particular those with injuries located at the bottom of the colony. Most of the colonies from S2 with Type 1 damages suffered an additional extension of damage, due to four uncontrolled factors: sedimentation, macroalgae overgrowth, Black Band Disease (BBD) and parrotfish bites (Fig. 5 ).
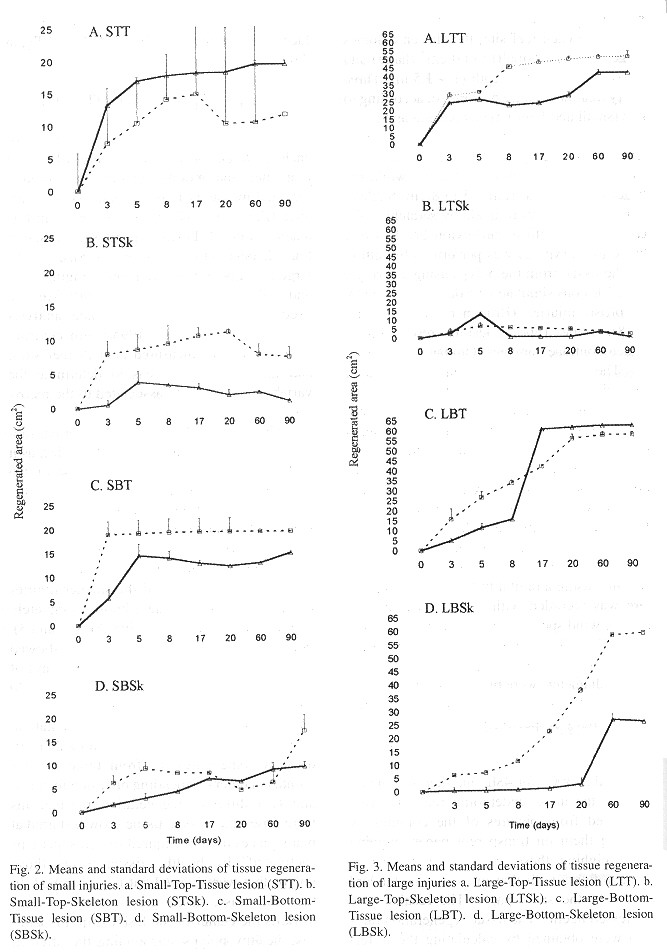
The regeneration rates ranged from 1 to 9cm 2/day; the fastest values were obtained for SI colonies under conditions, where the turbidity values were low. The minimum values were found at S2, a reef site with higher turbidity. Type 2 lesions showed a fast tissue growth, while Type 1 lesions showed lower values ( Table 1 ). A further observed trend was the decrease of tissue regeneration rates in time, as the maximum values were obtained during the first days of monitoring. The characteristies of lesions (size, type and position), reef site (locality) and time, significantly affected regenerated areas (Anova p< 0.001). More over, most of the interactions between factors resulted statistically significant (Anova p<0.01) (Table 2 ).
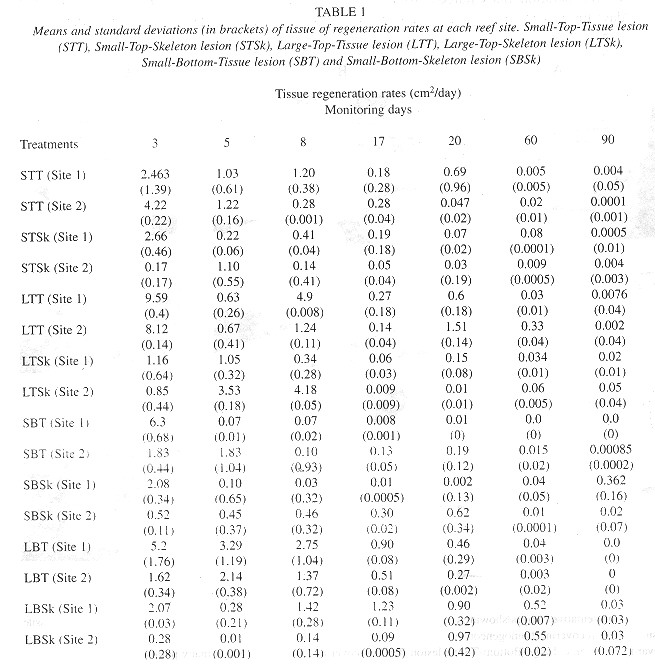
Environmental parameters: During the course of these experiments, the surface water temperature remained similar for both sites and ranged from 26 to 31ºC; the maximum temperatures were recorded late in the afternoon while the minima were recorded early in the morning, and air temperatures showed the same trend. The average of water turbidity measured at S2 was 2.303 ntu, whereas the average of recordings at SI was 1.711 ntu. Sedimentation rates were much higher at S2 (0.266g/cm 2/day) that at compared to SI (0.16g/cm2/day), while resuspension was low at SI (0.157g/cm2/day) in comparison with S2 (0.23g/cm 2/day). The test sites salinity showed little variations, ranging from 37 to 38% according to the time of day (Table 3 ).
Discussion
The regeneration of polips is a very important survival factor for corals, as this ability affects colony growth (Bak 1983 ), reproduction, the resistance to some diseases and the competitive performance of the colony (Meesters et al. 1994 ). Sedimentation is an important abiotic factor controlling growth, survival and development of coral reef communities (Rogers 1990 , Rielg 1995 , Kleypas 1996 ). The accumulation of sediment on damaged areas negatively affects the recovery from injuries, as Meesters et al. (1996) have demonstrated that the regeneration capacity of artificially inflicted lesions in the species Acropora palmata and M. annularis diminishes in areas where sedimentation rates are high. Sedimentation might affect lesion recovery because it induces major energy expenses in mucus production, sediment rejection and defense against algae overgrowth.
The environmental parameters measured at both reef sites during our study also were important factors in controlling the process of tissue regeneration. Injuries on corals from SI always remained free of silt, because the strong currents at this site inhibited sediment accumulation over the injuries. Colonies covered by silt frequently showed an extension of damage by BBD, and the fast bacterial growth might be related to these mortality factors, but further work involving both tissue regeneration and bacterial growth studies is required in order to corroborase this view.
The position of the wound on the colony was an important factor related to tissue regeneration. Meesters et al. (1996) found that injuries located at the top of colonies recovered faster than those located at the bottom. They suggested that polyps located at the top of the colony were healthier as compared to those located at the bottom, because the latter surrounded by sediments. Our data do not support this view, in as such as sediments usually covered top lesions and their regeneration was prevented, while bottom lesions remained elean because sediments did not accumulate over them, thus enhancing lesion recovery.
The type of lesions was also a key factor in the success of tissue regeneration seemingly, Type 2 lesions (Chactodontidae bites) recovered faster than those of Type 1 (Parrotfish bites) due lo the following reasons: first, when tissue is partially removed, new tissue layers begin to grow rapidly, probably relying on mechanisms of cellular division (Meesters and Bak 1993, Meesters et al. 1997). Secondly, if skeleton structures are destroyed, then the final recovery involves both the regeneration of tissue and the recovery of the skeletal structure. This involves a greater energetic cost or expenditure than in the former case.
Our findings indicate that BBD and parrotfish bites were the most important factors related to the extension of damages on the coral's surface. Many of the injuries located on the top of the colonies showed new bites of different sizes (60 x 70 mm to 10 x 12 mm) presumably caused by parrotfish (Scaridae) and surgeon fishes (Acanthuridae), respectively. When a colony is attacked by a parrotfish, other fishes also bite at the same injured colony, and this behavior accelerates the fast spread of damage and it may ultimately lead the to death of the entire affected colony in a relatively short time, Similar behavior patterns have been describes by Bruckner and Bruckner (1998) in Puerto Rico and other Caribbean locations.
Substrate competition has been recognized as one of the main biotic factors regulating the reef community structure (Connel 1973 , Lang 1973 , Rogers 1993 , Guzmán et al. 1994 , Tanner 1995 ). The mechanisms, by which competition reduces the coral fitness are still not very clear. However, it is known that diminished coral growth is related to the secretion of allopathic substances by algae along contact places between them and the corals (Tanner 1997 ). The reproduction and fecundity of the latter can decrease by abrasion, or by the continuos contact between polyps and macroalgae, which causes that polyps always remain retracted (Tanner 1997 ).
Based on our results, it emerges that the species M. annularis has the ability to regenerate damaged tissue fairly rapidly; however, the regeneration rates decrease almost exponentially with time. The regeneration of damages is strongly influenced by the characteristics of the lesion (size, type and position). The environmental parameters to which the colonies are normally exposed are a key factor for the final damage recovery. In our study case, clearly the main threats that a colony faces when it becomes damaged are overgrowth (especially by filamentous algae), extension of the damage by diseases (BBD) or fish bites, and damage by sedimentation. Our results suggest that the colonies of M. annularis have a greater probability of recovering from injuries that only involve the partial loss of tissue; this damage is comparable to that made by the bites of butterfly fishes. Lesions that imply both destruction of skeletal structures and tissue loss, take more time to recover. These damages are analogous to those produced by parrotfish bites.
Acknowledgments
We would like lo thank to Econatura, Fundación Científica Los Roques and Juan Carlos Femández, for all the logistics and financial support. I also want lo thank Héctor Guzmán, Jesús Ramos, Luis Bulla, David Bone, Sheila Marques Pauls, Roberto Cipriani and Percy Sanders, for their comments on the manuscript. Finally we thank the support of the staff (Pablo Mata, Francisco León, Jesús León and Melchor) of Dos Mosquises Sur Biological Station.
Resumen
En este estudio se determinó la tasa de regeneración de tejidos y la recuperación de colonias de Montastraea annularis, expuestas a diferentes grados de sedimentación después de inducir daños que simulan los mordiscos de peces mariposa y peces loro. El estudio se realizó en dos pequeños parches de arrecife escogidos cerca del Cayo Dos Mosquises, al norte de Venezuela. Dieciséis colonias (8 tratamientos + una réplica) fueron dañadas artificialmente en cada parche, y su recuperación fue monitoreada mediante fotografías durante tres meses. Las lesiones se produjeron usando dos técnicas: raspado de los pólipos, para semejar los daños de peces loro (Scaridae) (tipo 1) y aspiración del tejido para simular los mordiscos de peces mariposa (Chaetondontidae) (tipo 2). El diámetro de las lesiones varió entre 5 (lesiones pequeñas) y 8 cm (lesiones grandes) y ambos tipos fueron infringidos en partes de abajo y arriba de las colonias. Los principales factores que afectaron la recuperación de la superficie de las colonias fueron las características de la lesión (tipo, posición y tamaño), la turbidez y principalmente, la tasa de sedimentación. La recuperación de las lesiones fue lenta donde las tasas de sedimentación y resuspensión fueron altas, y la regeneración del tejido fue mejor en condiciones de baja sedimentación y resuspensión. Además, las lesiones localizadas en la parte inferior de las colonias se regeneraron completamente, en tanto que los sedimentos frecuentemente cubrieron las lesiones superiores y limitaron su recuperación. Más del 60% de las colonias con lesiones pequeñas se recuperaron casi completamente en menos de 90 días, mientras que aquellas con grandes heridas mostraron extensiones de sus áreas dañadas y aumentó su mortalidad. Las lesiones que afectaron solamente al tejido (tipo 1) se regeneraron 2 a 3 veces más rápido que aquellas que involucraron tanto al tejido y como al esqueleto (tipo 2).
References
Amend, T. 1992. Parque Nacional Archipiélago de los Roques, Serie Parques Nacionales y Conservación Ambiental. Tomo 3, Torino, Caracas. 223 p. [ Links ]
Bak, R.P.M. 1983. Neoplasia, regeneration and growth in the reefs building coral Acropora palmata. Mar. Biol. 77: 221-227. [ Links ]
Bak, R.P.M., J.W. Brounds & F.M.L Heys. 1977. Regeneration and aspects of spatial competition in the scleractinian corals Agaricia agaricites and Montastraea annularis. Proc. 31 Coral Reef Symp., Miami. pp. 143-148. [ Links ]
Bak, R.RM. & S. Van Es. 1980. Regeneration of superficial damage in the scleractinean coral Agaricia agaricites, Favia purpurea and Porites astreoides. Bull. Mar. Sci. 30: 883-887. [ Links ]
Brown, B.E. 1987. World wide death of corals- natural cyclical events or man made pollution? Mar. Poll. Bull. 18: 9-13. [ Links ]
Brown, B.E. 1990. Bleaching. Coral Reefs 8 (4): 135-232, [ Links ]
Bruckner, A. & R.J. Bruckner 1998. Rapid Wasting Syndrome or coral predation by spotlight parrotfish? Reef Encounter Newsletter (ICRS) 23: 18-26. [ Links ]
Connell, J.H. 1973. Population ecology of reefs building corals. pp. 205-245. In Jones O.A. & R. Endean (ed.). Biology and Geology of Coral Reefs. New York. [ Links ]
Foster, A.B. 1979. Phenotypic plasticity in the reefs corals Montastraea annularis and Siderastrea siderea. J. Exp. Mar. Biol. Ecol. 39: 25-54. [ Links ]
Guzmán, H.M., K.A. Burns, & J.B.C. Jackson. 1994. Injury regeneration and growth of Caribbean reef corals after a major oil spill at Panamá. Mar. Ecol. Prog. Ser. 105: 231-241. [ Links ]
Hall, R.V 1997. Interspecific differences in the regeneration of artificial injuries on scleractinean corals. J. Exp. Mar. Biol. Ecol. 212: 9-23. [ Links ]
Hayes R.L. & P.G. Bush. 1990. Microscopic observations of recovery in the reefs building scleractinean coral Montastraea annularis after bleaching en a Cayman reef. Coral Reefs 8: 203-209. [ Links ]
Harvell, C.D, K. Kim, J.M. Burkholder, R.R. Colwell, P.R. Epstein, D.J. Grimes, E. E. Hofmann, E.K. Lipp, A.D. M.E. Osterhaus, R. M. Overstreet, J.M. Porter, G.W. Smith & G.R. Vasta. 1999. Emerging Marine Diseases - Climate Links and Anthropogenic factors. Science 285: 1505-1510. [ Links ]
Hung, M. 1985. Los corales pétreos del Parque Nacional Archipiélago Los Roques. Tesis de Licenciatura, Universidad Central de Venezuela, Venezuela. 204 p. [ Links ]
Jongman, R.H. G., C.J.F. Ter Braak & O.F.R. Van Tongeren. 1995. Data Analysis in Community and Landscape Ecology. University, Cambridge. 292 p. [ Links ]
Kleypas, J.A. 1996. Coral reef development under naturally turbid conditions: fringing reefs near Broad Sound, Australia. Coral Reefs 15: 153-167. [ Links ]
Knowlton, N., J.L. Maté, H.M. Guzmán & R. Rowan. 1997. Direct evidence for reproductive isolation among the three species of the Montastraea annularis complex in Central America (Panamá and Honduras). Mar. Biol. 127: 705-711. [ Links ]
Lang, J. 1973. Interspecific aggression by seleractincan coral. 2. ¿Why the race is not only to the swift? Bull. Mar. Sci. 23: 260-279. [ Links ]
Lester, R.T. & R.RM. Bak. 1985. Effect of environment on regeneration of tissue lesions in the reef coral Montastraea annularis (Scleractinea). Mar. Ecol. Prog. Ser. 24: 183-185. [ Links ]
Loya, Y 1976. Recolonization of Red Sea corals affected by natural catastrophes and man made perturbations. Ecology 57: 278-279. [ Links ]
Meesters, E.H. & R.RM. Bak. 1993. Effects of coral bleaching on tissue regeneration potential and colony survival. Mar. Ecol. Prog. Ser. 96: 189-198. [ Links ]
Meesters, E.H., M. Noordeloos, & R.P.M. Bak. 1994. Damage and regeneration: links to growth in the reef building coral Montastraea annularis. Mar. Ecol. Prog. Ser. 112: 119-128. [ Links ]
Meesters, E.H. & R.P.M. Bak. 1995. Age-related deterioration of a physiological function in the branching coral Acropora palmata. Mar. Ecol. Prog. Ser. 121: 203-209. [ Links ]
Meesters, E.H., I. Wesseling & R.P.M. Bak. 1996. Partial mortality in three species of reef building corals and its relation with colony morphology. Bull. Mar. Sci. 58: 838-852. [ Links ]
Meesters, E.H., W. Pauchli & R.P.M. Bak. 1997. Predicting regeneration of physical damage on a reef - building coral by regeneration capacity and lesion shape. Mar. Ecol. Prog. Ser. 146: 91-99. [ Links ]
Nagelkerken, I. & R.P.M. Balc. 1998. Differential regeneration of artificial lesions among sympatric morphs of the Caribbean corals Porites astreoides and Stephanocoenia michelinii. Mar. Ecol. Prog. Ser. 163: 279-283. [ Links ]
Ohman, M.C., A. Rajasuriya & S. Sevensson. 1998. The use of Butterflyfishes (Chaetodontidae) as bio - indicators of habitat structure and human disturbance. Ambio 27(8): 708-716. [ Links ]
Oren, U., Y Benayahu & Y. Loya. 1998. Effect of lesion size and shape on regeneration of the Red Sea coral Faviafavus. Mar. Ecol. Prog. Ser. 146: 101-107. [ Links ]
Rielg, B. 1995. Effects of sand deposition on scleractinean and alcyonacean corals. Mar. Biol. 121: 517-526. [ Links ]
Rogers, C. 1990. Responses of coral reef organisms to sedimentation. Mar. Ecol. Prog. Ser. 62: 185-202. [ Links ]
Rogers, C. 1993. Hurricanes and coral reefs: the intermediate disturbance hypothesis revisited. Coral Reefs 12: 127-137. [ Links ]
Reusik, L.J. 1997. Coral injury and recovery: matrix models link process to pattern. J. Exp. Mar. Biol. Ecol. 210: 187-208. [ Links ]
Tanner, J.E. 1995. Competition between scleractinean corals and macroalgae: An experimental investigation of coral growth, survival and reproduction. Jour Exp. Mar. Biol. Ecol. 190: 151-168. [ Links ]
Tanner, J.E. 1997. Interspecific competition reduces fitness in scleractinean corals. J. Exp. Mar. Biol. Ecol 214: 19-34. [ Links ]
Van Veghel, M., F.R. Daniel, & R.P.M. Bak. 1996. Interspecific interactions and competitive ability of the polymorphic reef-building coral Montastraea annularis. Bull. Mar. Sci. 58: 708-803. [ Links ]
Weil, E. & N. Knowlton. 1994. A multicharacter analysis of the Caribbean coral Montastraea annularis (Ellis and Solander, 1786) and its two sibling species, M. faveolata (Ellis and Solander, 1786) and M. franksi (Gregory, 1895). Bull. Mar. Sci. 55: 151-175. [ Links ]
Woesik, R.V. 1998. Lesion healing on massive Porites spp. corals. Mar. Ecol. Prog. Ser. 164: 213-220. [ Links ]
Zar, H.J. 1998. Biostatistical Analisis. Prentice Hall, New Jersey. 663 p. [ Links ]
1 . Universidad Simón Bolivar Sartenejas, Edificio Básico 1, Laboratorio de Comunidades Marinas, Caracas, Venezuela; croquer@telcel.net.ve . Fax:02129063416.
2 . Fundación Científica Los Roques, Urbanización Country Club, Caracas, Venezuela.
3 . Universidad Central de Venezuela, Facultad de Ciencias. Instituto de Biología Tropical; museomar@telcel.net.ve














