Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.50 n.2 San José Jun. 2002
Francisco Kelmo 1 and Rita Vargas 2
1 Benthic Ecology Research Group, 606 Davy Building, University of Plymouth, Drake Circus, Plymouth, Devon, PL48AA. United Kingdom. E-mail: F.Kelmo@plymouth.ac.uk
2 Museo de Zoología, Escuela de Biología, Universidad de Costa Rica, 2060 San José, Costa Rica. E-mail: ritav@biologia.ucr.ac.cr
Received 16-IX-2000. Corrected 04-VI-2001. Accepted 30-V-2002.
Abstract: This paper is the first taxonomic account of the hydroid orders Anthoathecatae and Leptothecatae from the Caribbean and Pacific coast of Costa Rica. All specimens are currently stored at the reference collection of the Museo de Zoología, Escuela de Biología, Universidad de Costa Rica. Sixteen hydroid species are recorded: Eudendrium carneum, Pennaria disticha, Acryptolaria longitheca, Plumularia floridana, Halopteris polymorpha, Aglaophenia dubia, Aglaophenia latecarinata, Lytocarpia tridentata, Macrorhynchia phillipina, Macrorhynchia sp., Clytia gracilis, Cnidoscyphus marginatus, Thyroscyphus ramosus, Dynamena disticha, Sertularella diaphana, and Tridentata distans. An extensive synonymy has been given for each species. A simplified taxonomic key is included, and illustrations and descriptions are provided for each species.
Key words: Anthoathecatae, Lepthothecatae, hydroids, taxonomy, Costa Rica.
The hydroid fauna of Costa Rica is little studied and therefore poorly known. Studies on species from the Pacific coast of the country include those of Fraser (1938, 1948) and Hertlein (1963), but no study exists recording the hydroids from the Caribbean coast. The most recent report on the biodiversity of cnidarians from Costa Rica (Cortés 1997) is a list of species previously reported.
The scarcity of reliable taxonomic characters in various hydroid taxa, together with a scarcity of data on the biology of these animals, including the life cycles (Calder 1988, Kelmo and Santa-Isabel 1998), hampers hydrozoan taxonomy. This discourages research into the group, and therefore impedes the advancement of knowledge.
The purpose of this report is to provide the first taxonomic account of the Anthothecate and Leptohecatae hydroids currently stored at the reference collection of the Museo de Zoología, Escuela de Biología, Universidad de Costa Rica (UCR), San Pedro, San José, Costa Rica. Here we present a detailed synonymy, a simplified identification key, descriptions and illustrations for each studied species covering both Pacific and Atlantic coasts.
Materials and methods
Colonies were examined using stereoscopic and light microscopy, and the taxonomic identifications were based on the specific literature noted in the text of this paper. All descriptions provided herein are from the examined material, with accompanying illustrations constructed using brightfield microscopy.
The listed synonymy essentially follows that of Calder (1988, 1991, 1997a), Marques (1992), Migotto (1993), and Kelmo and Santa-Isabel (1998), and virtually all of these lists have been verified by examination of the original references. In most of cases, only one significant record has been cited to document occurrences worldwide. Hydroid classification has been extensively modified by Petersen (1979, 1990), Werner (1984), Bouillon (1985), and Calder (1988, 1991, 1997a). However, in the interests of simplicity, the classification adopted here is that followed by Calder (1988, 1991, 1997a, 1997b).
Nematocysts were examined by compressing pieces of tissue, or entire individuals, between slide and coverslip (Calder 1991). The specimens were treated with 10% solution of sodium hypoclorite for 5-10 seconds, and rinsed twice in distilled water prior to slide preparation (Kelmo and Santa-Isabel 1998). Nematocyst categories were identified according to the classification of Weill (1934). At least 10 nematocysts of each type were measured from each species in order to determine size ranges.
Results
A total of 243 colonies were analyzed, consisting of nine families and sixteen species of anthoathecate and leptothecate hydroids from both the Pacific and Caribbean coast of Costa Rica (Table 1).
Order Antoathecatae Cornelius, 1992
Super Family Eudendrioidea L.Agassiz, 1862
Family Eudendriidae L. Agassiz, 1862
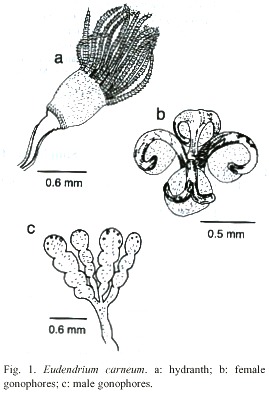
Eudendrium carneum Clarke, 1882
Fig. 1)
Eudendrium ramosum McCrady, 1859; A.Agassiz, 1865; Congdon, 1906; Fraser, 1912; Bennitt, 1922 [ Not Eudendrium ramosum (Linnaeus, 1758)]. Eudendrium carneum Clarke, 1882; Calder, 1988; Marques, 1992; Kelmo and Santa-Isabel, 1998. Eudendrium cunninghami Kirkpatrick, 1910.
Material examined: eleven colonies, with male and female gonophores, Puerto Limón, Limón, Caribbean coast, 9°5940 N, 83°0130 W, 12 November 1993 (UCR-845).
Description: branched and bushy colonies, up to 12 cm, arising from creeping hydrorhizal stolons. Hydrocaulus upright, polysiphonic, more or less alternately branched; primary branches also polysiphonic and irregularly branched; secondary branches polysiphonic basally and irregularly or alternately branched. Perisarc thick, annulated or wrinkled at bases of branches and hydranth pedicels, with occasional annulations elsewhere. Hydranths urn-shaped, about 0.82 mm long from proximal end to base of hypostome, 0.63 mm wide, with a shallow perisarc groove; hypostome very large, flared to knob-shaped. Tentacles solid, filiform, 28-32 in number, disposed in one whorl. Gonophores fixed sporosacs, developing on hydranth distal to perisarc groove. Female gonophores on reduced hydranths with partially atrophied tentacles; spadix bifid, curving over egg. Male gonophores with up to five chambers each, borne on atrophied hydranths. Cnidome comprising heterotrichous anisorhizas 20.2-22.8 µm x 9.3-11.4 µm, and by heterotrichous microbasic euryteles 8.2-9.4 µm x 3.5-4.0 µm.
Known range: Bermuda (Congdon 1906, 1907; Bennitt 1922; Summers 1972; Calder 1986, 1988); western Atlantic (Fraser 1944); eastern Atlantic (Kirkpatrick 1910); Indian Ocean (Millard 1975); eastern Pacific, Gulf of California, north of Clarion Island (Fraser 1948); Brazil (Marques 1992, Kelmo and Santa-Isabel 1998).
Family Halocordylidae Stechow, 1921
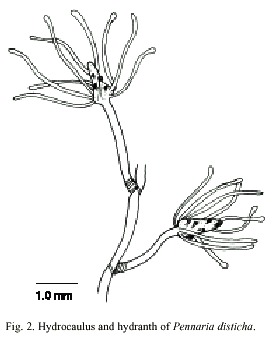
Pennaria disticha (Goldfuss, 1820)
(Fig. 2)
Pennaria disticha Goldfuss, 1820; Plumularia pennaria Blainville, 1830; Pennaria cavolini Ehrenberg, 1834; Chiaje, 1841 in Calder, 1988. Anisocalyx pinnarium Costa, 1842. Globiceps tiarella Ayres, 1854. Eucoryne elegans Leidy, 1855. Pennaria tiarella McCrady, 1859. Pennaria gibosa L.Agassiz, 1860. Halocordyle tiarella Allman, 1872. Pennaria symmetrica Clarke, 1879. Globiceps globator Haeckel, 1879. Pennaria inornata Brooks, 1883. Pennaria australis Bale, 1884. Pennaria adamsia van Lendenfeld, 1885b. Pennaria pennaria Marktanner-Turneretscher, 1890. Halocordyle australis Bale, 1894. Halocordyle cooperi Warren, 1906. Pennaria pacifica Clarke, 1907. Pennaria australis var. cooperi Warren, 1908. Pennaria australis Warren, 1908. Pennaria disticha var. australis Ritchie, 1910. Pennaria wilsoni Bale, 1913. Halocordyle disticha Stechow, 1923a. Halocordyle wilsoni Stechow 1923a. Corydendrium splendidum Boone, 1938. Halocordyle disticha var. australis Vervoort, 1941. Halocordyle fragilis Vannuci, 1951. Halocordyle pennaria var. australis Mammem,1963. Pennaria americana García-Corrales and Aguirre, 1985 [Nomem Nudum according to Calder (1988)]. Penaría (Halocordyle) tiarella García-Corrales and Aguirre, 1985. Pennaria europea García-Corrales and Aguirre, 1985 [Nomem Nudum according to Calder (1988)]; Pennaria (Halocordyle) disticha García-Corrales and Aguirre, 1985. Pennaria symetrica García-Corrales and Aguirre, 1985; Halocordyle disticha Calder, 1988; Kelmo and Santa-Isabel, 1998; Pennaria disticha Silveira and Migotto, 1992.
Material examined: four colonies without gonophores or medusae buds, artificial reef, Bahía Huevos, Guanacaste, Pacific coast, 10°3840 N, 85°4130 W, 28 March 1984 (UCR-895). Eight colonies without gonophores or medusae buds, Manzanillo, Limón, 9º 3810 N, 82º 3810 W, Caribbean coast, 19 September 1992 (UCR-690).
Description: erect colonies, arising from a creeping and branched hydrorhiza; growth monopodial with terminal hydranths. Hydrocaulus monosiphonic, reaching about 0.4 mm wide, zigzag to nearly straight, annulated basally, divided at more or less regular intervals by one or more well-developed annulations; internodes 0.62-4.0 mm long, each typically supporting a branch distally. Perisarc thick, black or dark-brown. Branches up to 28 mm long, annulated basally, given off alternately from opposite sides of hydrocaulus, curved outwards, divided into internodes. Internodes 1.6-4.2 mm long, marked by distinct to rather faint annulations proximally and distally; each internode giving rise to a ramulus from both its upper surface and its distal end. Ramuli unbranched, annulated basally or throughout entire length, each terminating in a hydranth. Hydranths clavate to pear-shaped, up to 1.8 mm long, 0.35 mm wide; with a whorl of about 10 to 18 long, filiform or faintly knobbed tentacles aborally; a varied number of short, capitate tentacles in one or more regular or irregular verticils medially; and a whorl of about five capitate tentacles orally. Hypostome dome shaped. Cnidome comprising desmonemes 4.4-5.3 µm x 3.2 -3.8 µm; basitrichous haplonemes 5.6-9.5 µm x 2.5- 3.7 µm; heterotrichous microbasic euryteles 10.6-13.5 µm x 6.0-6.9 µm, and four sizes of stenoteles: (i) very small, 5.5-6.5 µm x 4.2- 4.6 µm; (ii) small, 7.3-7.8 µm x 5.4-5.8 µm; (iii) medium, 14.1-17.5 µm x 10.0-12.2 µm, and (iv) large, 28.0-40.0 µm x 16.4-20.5 µm.
Known range: Bermuda (Verrill 1900, Congdon 1907, Bennitt 1922, Weill 1937, Cowden 1964, 1965a, b; Summers and Haynes 1969; Summers 1970, 1972; Lesh-Laurie 1976, Calder 1986, 1988); weastern Atlantic (Fraser 1944); eastern Atlantic (Brinckmann-Voss 1970); Indian Ocean (Millard 1975); western Pacific (Yamada 1959); eastern Pacific, from San Pedro, California; San Francisco Bay, east of Panamá City; off La Libertad, Ecuador (Fraser 1948); Brazil (Migotto 1993, Kelmo and Santa-Isabel 1998).
Order Leptothecatae Cornelius, 1992
Superfamily Lafoeoidea A.Agassiz, 1865
Family Lafoeidae A.Agassiz, 1865
Subfamily Lafoeinae A. Agassiz, 1862
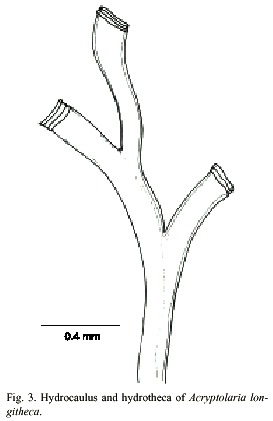
Acryptolaria longitheca (Allman, 1877)
(Fig. 3)
Cryptolaria longitheca Allman, 1877; Clarke, 1879; Fewkes, 1881; Bedot, 1912; Bedot, 1916; Bedot, 1918; Bedot, 1925; Cryptolaria crassicaulis Driesch, 1889; Ritchie, 1911; Leloup, 1932; Stranks, 1993. Lafoea (Cryptolaria) longitheca Bonnevie, 1899; Billard, 1906. Cryptoaria crassicaulis Pictet and Bedot, 1900; Browne, 1907; Jaderholm, 1907; Stechow, 1913a, b; Jaderholm, 1919. Cryptolaria crassicaulis var. dimorpha Ritchie, 1911; Jarvis, 1922. Oswaldaria crassicaulis Stechow, 1921; Stechow, 1923b; Leloup, 1940. Oswaldaria longitheca Stechow, 1923b; Acryptolaria longitheca Fraser, 1943, 1944; Deevey, 1954; Vervoort, 1968; Vervoort, 1972; Cairns et al., 1991; Calder, 1996; Calder and Vervoort, 1998. Acryptolaria crassicaulis Yamada, 1959; Rees and White, 1966; Millard, 1967; Leloup, 1974; Gravier-Bonnet, 1979; Vervoort, 1985; Ramil and Vervoort, 1992; Altuna Prados, 1994; Blanco, 1994a,b; Altuna Prados, 1995; Bouillon et al., 1995; Mendel and López Gonzalez, 1996. Acryptolaria crassicaulis var dimorpha Smaldon et al., 1976; Gravier-Bonnet, 1979.
Material examined: one colony without gonothecae, northwestern Isla del Caño, Puntarenas, Pacific coast, 8°4608 N, 84°1704 W, 990 m, 25 January 1994 (UCR-899).
Description: colony erect and flabellate, 1.2 cm high, arising from a creeping hydrorhiza. Hydrocaulus geniculate, polysiphonic; nodes indistinct; branches in one plane, resembling hydrocaulus, and not secondarily branched. Perisarc fairly thick. Hydrothecae very long, slender, total length excluding renovations is about 1793 µm, occurring on hydrocaulus and branches, given off alternately in one plane, each adnate to axial tube for most of its length, curving outwards and slightly frontwards, becoming free distally; narrowest at the base, gradually widening distally, free part nearly cilyndrical. Hydrothecal walls smooth; perisarc of abcauline wall thicker basally than distally. Margin entire, slightly flaring, usually with 3 or 4 renovations; orifice round, diameter about 296 µm. Operculum, diaphragm, desmocytes and nematothecae absent. Cnidome represented by very small microbasic mastigophores, 3.5–4.2 µm x 1.1–1.4 µm.
Known range: western Atlantic, mid-Atlantic Ridge, Australia (Allman 1877, 1888 as Cryptolaria crassicaulis); Clarke 1879, Vervoort 1972, Calder 1996, Calder 1997b, Calder and Vervoort 1998.
Family Thyroscyphidae Stechow, 1920
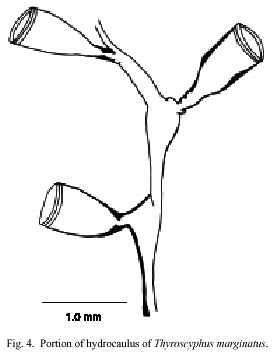
Thyroscyphus marginatus (Allman, 1877)
(Fig. 4)
Obelia marginata Allman, 1877; Campanularia insignis Allman, 1888 [not Campanularia insignis Fewkes, 1881 = Campanularia macroscypha Allman, 1877]; Obelia (Lytoscyphus) marginata Ritchie, 1909; Lytoscyphus insignis Ritchie, 1909; Lytocysphus marginata Ritchie, 1909; Lytoscyphus marginatus Billard, 1910; Campanularia marginata Nutting, 1915; Leptocysphus marginatus Jaderholm, 1920; Cnidoscyphus marginatus Splettsosser, 1929; Thyroscyphus marginatus Calder, 1983, 1990.
Material examined: twelve colonies without gonophores, Manzanillo, Limón, Caribbean coast, 9º 3755 N, 82 o 3910 W, 19 September 1992 (UCR-688). Nineteen colonies without gonophores, Punta Piuta, Limón, Caribbean coast, 10°0035 N, 83°0215 W, subtidal zone, 1-2 m, 13 November 1983 (UCR-847).
Description: colonies erect, up to 20 cm high, arising from a creeping hydrorhiza. Hydrocaulus monosiphonic, up to 1 mm wide, straight basally, becoming slender and distinctively geniculate distally, divided into internodes at regular intervals by slightly, distinct or indistinct, oblique nodes. Perisarc thick basally and thinner distally. Internodes with prominent apophysis distally; apophyses alternating from side to side, each supporting a hydrotheca. Hydrocladia typically alternate, unbranched or alternately branched; hydrocladia divided by oblique nodes into internodes resembling, but more slender than, internodes of hydrocaulus, and with basal internode somewhat longer than others. Hydrothecae pedicellate, cone-shaped, 1018-1090 µm long from apophysis to margin; adcauline wall convex, abcauline wall nearly straight; diaphragm a ring of perisarc, often somewhat thicker on adcauline than abcauline wall; margin entire, occasionally renovated, with a ring like thickening; hydrothecal operture round, 486- 680 µm wide; operculum shed. Hydranth attached to hydrothecal wall by an annular fold distal to diaphragm. Abcauline diverticulum not observed. Gonophores not observed. Cnidome comprising microbasic mastigophores of three distinct sizes: (i) very large, 31- 34 x 12.4-16 µm; (ii) large, 27-29 x 9.8- 10.6 µm; and (iii) small, 3.5-3.9 x 1.1-1.8 µm.
Known range: Bermudas (Allman 1888, Congdon 1907, Ritchie 1909, as Campanularia insignis), (Jaderholm 1920 as Leptoscyphus marginatus), (Bennit 1922, Fraser 1944 as Campanularia marginata), Calder 1986, 1990; western Atlantic Calder 1983; eastern Atlantic Vervoort 1959.
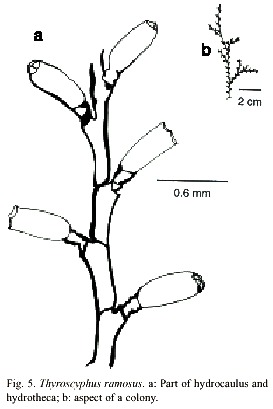
Thyroscyphus ramosus (Allman, 1877)
(Fig. 5)
Thyroscyphus ramosus (Allman, 1877); (Splettstösser, 1929); (Vervoort, 1959); (van Gemerden-Hoovegeen, 1965).
Material examined: thirteen colonies, without gonophores, Punta Moín, Limón, Caribbean coast, 10°0005 N, 83°05W, 9 m, 12 November 1983 (UCR-842). Nine colonies without gonophores, north-east coast of Isla Uvita, Limón, Caribbean coast, 9°5940 N, 83°0050 W, 11 November 1993 (UCR-844). Eight colonies without gonophores, Refugio Nacional de Vida Silvestre Gandoca-Manzanillo, Limón, Caribbean coast, 9°3755 N, 82°3910 W. 20 m, 4 August 1995 (UCR-849).
Description: colonies erect, up to 25 cm high, arising from a creeping hydrorhiza. Hydrocaulus monosiphonic, up to 1.2 mm wide, divided into internodes, by oblique nodes. Internodes of 1180-2968 µm long and 250-772 µm in diameter, inclined alternately in opposite directions. Nodes vary from 300 to 1043 µm in diameter. Irregularly branched. Perisarc of moderate thickness. Internodes with an apophysis distally placed, supporting a pedicellate hydrotheca. Pedicel short, with 2 to 5 annulations. Hydrothecae alternately placed; abcaulinar wall slightly convex. Apophysis margin of hydrothecae about 1198 to 1802 µm long, and diaphragm margin vary from 800 to 1343 µm long. Diaphragm oblique, virtually thicker on adcauline wall. Margin with four teeth of moderate prominence. Operculum with four conspicuous valves. Gonothecae not observed. Cnidome comprising microbasic mastigophores of two distinct sizes: (i) very large, 22.6–25.2 x 10.3–11.8 µm and (ii) small, 8.1–9.6 x 3.0–4.1 µm.
Known range: Caribbean Sea (Van Germerden-Hoogeveen 1965); western Atlantic (Vervoort 1959); southern Atlantic (Allman 1888, Migotto 1993).
Family Sertulariidae Lamouroux, 1812
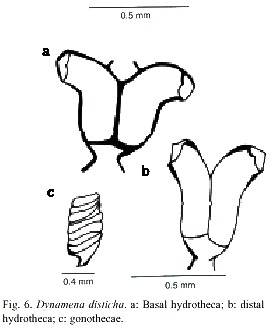
Dynamena disticha (Bosc, 1802)
(Fig. 6)
Sertolara pumila Cavolini, 1785; Sertularia disticha Bosc, 1802; Dynamena disticha Lamouroux, 1812; Dynamena cornicina McCrady, 1859; Sertularia cornicina Verrill, 1874; Sertularia exigua Allman, 1877; Sertularia complexa Clarke, 1879; Sertularia mayeri Nutting, 1904; Sertularia pourtalesi Nutting, 1904; Sertularia densa Stechow, 1919; Dynamena densa Stechow, 1920; Tridentata complexa Stechow, 1922; Tridentata cornicina Stechow, 1922; Tridentata disticha Stechow, 1922; Tridentata exigua Stechow, 1922; Tridentata pourtalesi Stechow, 1922; Dynamena mayeri Stechow, 1925; Dynamena disticha form dense Picard, 1951a; Dynamena disticha form souriei Picard, 1951b; Dynamena cavolini Riedl, 1959 [Justified emendation]; Dynamena disticha Calder, 1990; Migotto, 1993; Souza, 1997.
Examined material: thirty six colonies with gonothecae, northeast part of Isla Uvita, Limón, Caribbean coast, 9°5940 N, 83°0050 W, 11 November 1993 (UCR-844).
Description: colonies erect, up to 1.2 cm high, arising from a creeping hydrorhiza. Hydrocaulus monosiphonic, straight, unbranched, divided into internodes by usually distinct oblique nodes, or by distinct hinge-joints; basal part of hydrocaulus with one or more athecate internodes 1.6 to 2.1mm long, each with one pair of frontally placed hydrothecae. Hydrothecal pairs contiguous for a varying distance in front of internode; distal half parallel with axis of internode; distal half curving outwards with orifice facing nearly perpendicular to internodal axis. Abcauline wall concave, 267-474 µm long; contiguous portion of adcauline wall straight, 129-360 µm long; free part of adcauline wall convex, 202- 350 µm long. Hydrothecal base about 112 µm wide, with triangular perisarcal projections extending proximally; hydrothecal orifice oval, 98-136 µm wide from adcauline to abcauline wall; margin with two large pointed lateral teeth and a smaller marginal adcauline tooth. Operculum consisting of a large abcauline valve and a smaller tent-shaped adcauline valve. Hydrothecae usually with internal teeth. Gonophores fixed sporosacs. Gonothecae oval-shaped laterally, round-shaped in cross section, usually with six, rounded, tranverse ridges. Gonotechae about 1mm long from base to orifice, 0.62 mm wide, placed by short pedicels into hydrorhiza. Submarginal teeth present. Cnidome comprising microbasic mastigophores of two distinct sizes: (i) large, 21.0–26.2 x 8.9–10.02 µm, and (ii) small, 6.8–8.1 x 2.0–2.8 µm.
Known range: Bermudas (Bennitt 1922 as Sertularia cornicina; Calder 1986, 1990), western Atlantic (García et al 1980), Indian Ocean (Mammen 1965), western Pacific (Park and Rho 1986 as Dynamena cornicina), eastern Pacific (Fraser 1937), Brazil (Migotto 1993, Souza 1997).
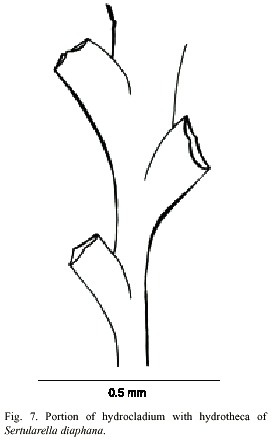
Sertularella diaphana (Allman, 1885)
(Fig. 7)
Thuiaria distans Allman, 1877; Thuiaria pinnata Allman, 1877; Thuiaria diaphana Allman, 1885; Thuiaria hyalina Allman, 1888; Sertularella distans Hartlaub, 1901; Sertularella pinnata Hartlaub, 1901; Thujaria pinnata Hartlaub, 1901b; Sertularella pinigera Hartlaub, 1901b; Sertularella lata Nutting, 1904; Sertularella lata Billard, 1907, Jarvis, 1922. Sertularella torreyi Nutting, 1905; Sertularella speciosa Congdon, 1907; Sertularella tridentata Stechow, 1913a; Sertularella diaphana Bale, 1919; Sertularella delicata Billard, 1919; Sertularella sargassi Stechow, 1920; Sertularella diaphana madagascariensis Billard, 1921; Sertularella diaphana orthogona Billard, 1921; Thuiaria quadrilateralis Hargitt, 1924; Sertularella diaphana var gigantea Billard ,1925a; Sertularella diaphana var delicata Billard, 1925. Sertularella diaphana Calder, 1990, Souza, 1997.
Examined material: five colonies without gonothecae, Refugio Nacional de Vida Silvestre Gandoca-Manzanillo, northern Punta Mona, Limón, Caribbean coast, 9°3745 N, 82°3707 W, 9 m, 4 August 1995 (UCR-846).
Description: colonies erect, up to 16 mm high, arising from a creeping hydrorhiza. Hydrocaulus usually polysiphonic, rarely monosiphonic, slightly geniculate, divided into internodes at regular intervals by oblique nodes sloping alternately left and right. Perisarc thick. Internodes with pairs of alternate hydrothecae; apophyses short, given off alternately from opposite sides of hydrocaulus; each apophysis separated from hydrocladium by a distinct transverse node. Hydrocladia straight to slightly geniculate, up to 9.3 mm long, divided into internodes of varied length by oblique nodes. Hydrothecae alternate, on opposite sides of hydrocladia, almost entirely adnate, each with axis oblique to that of internode; adcauline wall convex, 310-349 µm long; abcauline wall slightly concave, occasionally with a faint bulge basally; basal diameter 105- 151 µm. Hydrothecal orifice nearly circular, 201-220 µm wide from adcauline to abcauline tooth; margin with four small teeth. Operculum with four valves. Hydranths with an abcauline diverticulum. Gonophores not observed. Cnidome comprising microbasic mastigophores of two distinct categories: (i) large, 23.2–2 7.4 x 9.3–11.2 µm, and (ii) small, 6.6–8.4 x 2.6–3.1 µm.
Known range: Bermudas (Congdon 1907 as Sertularella speciosa, Calder 1990), western Atlantic (Vervoort 1968 as Sertularella speciosa), Indian Ocean (Millard 1975), western Pacific (Billard 1925a), Brazil (Souza 1997).
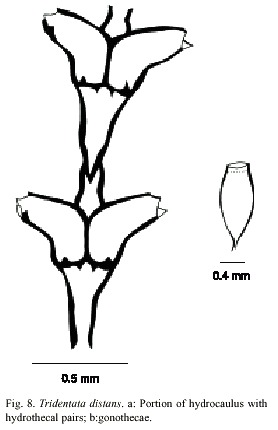
Tridentata distans (Lamouroux, 1816)
(Fig. 8)
Dynamena distans Lamouroux, 1816; Sertularia lamourousii Deshayes and Milne-Edwards, 1836; Sertularia gracilis Hassall, 1848; Sertularia (Dynamena) distans Busk, 1852; Dynamena gracilis Marktanner-Turneretscher, 1890; Sertularia stookeyi Nutting, 1904; Stechow, 1922. Sertularia distans Billard, 1906; Sertularia distans gracilis Billard, 1912; Tridentata gracilis Stechow, 1920; Tridentata (Sertularia) gracilis Stechow, 1920; Tridentata heterodonta Stechow, 1922. Tridentata distans Hiroito, 1969; Calder, 1990; Souza, 1997. Sertularia distans Migotto, 1993.
Examined material: twenty five colonies with gonophores, south of lighthouse, Isla del Caño, Puntarenas, Pacific coast, 8°43 N, 83°52 W, 19 April 1984 (UCR-892).
Description: colonies erect, up to 12 mm high, arising from a creeping hydrorhiza having internal septa. Perisarc thick. Hydrocaulus monosiphonic, straight, usually with a long stolon distally, unbranched but occasionally with one to four branches; each branch arising from renovation of an old hydrotheca, divided into internodes. Basal part of hydrocaulus with one to six short athecatae internodes separated by oblique hinge-joints. Upper thecate internodes 286-432 µm long, separated by distinct or indistinct oblique to occasionally almost transverse nodes; each internode with a distal pair of frontally placed hydrothecae. Sometimes two athecate internodes separated by a short athecate internode marked by an oblique node at proximal end and oblique hinge-joint at distal end. Hydrothecal pairs opposite; pairs virtually contiguous; rarely separated frontally. Hydrothecae horn-shaped, facing outwards to obliquely upwards, each with an axis oblique to that of internode; abcauline wall 180-232 mm long, concave medially, nearly straight distally, often with a basal bulge proximally; contiguous part of adcauline wall straight, 5- 160 µm long, becoming nearly straight distally; diameter at base 55-118 µm. Perisarc of hydrothecae thickened near margin; adcauline and abcauline walls each either lacking intrathecal teeth or having one or two such teeth just below margin; hydrothecal base near adcauline wall with a small triangular perisarcal projection extending proximally into internode, and usually with a perisarcal projection protruding from base near hydropore into hydrothecal cavity. Hydrothecal orifice irregularly oval, 60-90 µm wide from adcauline to abcauline wall; margin with two lateral teeth and a smaller median adcauline tooth. Operculum consisting of a large abcauline valve. Hydranth with an abcauline diverticulum. Gonophores fixed sporosacs. Gonothecae arising from short pedicels on hydrocaulus at bases of hydrothecae, normally numbering two per hydrocaulus, elongated and oval in lateral view, irregularly oval to nearly round in cross section, with about four faint, rounded ridges. Gonothecae vary from 923 to 1024 µm long from base to orifice; diameter vary from 398 to 414 µm. Orifice nearly round, about 220 µm in diameter. Operculum and submarginal teeth present. Cnidome comprising microbasic mastigophores of two distinct sizes: (i) large, 14.0–15.2 x 3.6–4.5 µm and (ii) small, 5.2–5.9 x 2.0–2.7 µm.
Known range: Bermuda (Bennit 1922 as Sertularia stookey, Calder 1991), western Atlantic (van Gemerden-Hoogeven 1965 as Sertularia distans var gracilis), eastern Atlantic (Cornelius 1979 as Sertularia distans), Indian Ocean (Millard 1975 as Sertularia distans), western Pacific (Hirohito 1969), eastern Pacific (Fraser 1948 as Sertularia stookey).
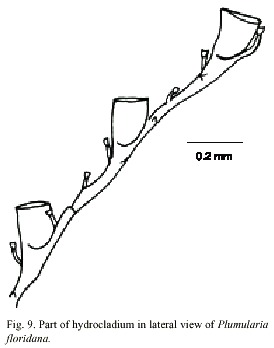
Plumularia floridana Nutting, 1900
(Fig. 9)
Plumularia floridana Nutting, 1900; Plumularia alicia Torrey, 1902; Plumularia florida Wallace, 1909; Plumularia alicia var. minuta Billard, 1927; Plumularia sinuosa Fraser, 1938; Plumularia floridina Pennycuik, 1959; Plumularia sp. Pennycuik, 1959; ?Plumularia indica Mammen, 1965; Plumularia pennycuikae Millard and Bouillon, 1973; Plumularia pennycuikai Rho and Park, 1986.
Material examined: one colony with several hydrocauli, gonophores not observed, Punta Pitahaya, Guanacaste, Pacific coast, 10º0355 N, 85º4557 W, 15 June 1991 (UCR-898).
Description: colony 12 mm high, with a creeping hydrorhiza. Hydrocaulus monosiphonic, branched, more or less straight basally, geniculate elsewhere, divided at regular intervals beyond basal region internodes by distinct transverse nodes; internodes 290-600 µm long, 40-90 µm wide at nodes, with conspicuous septa adjacent to nodes. Each internode with a distal apophysis and typically with two or more nematothecae, one or two axillary and one along internode on side opposite apophysis; cauline nemathothecae bithalamic, movable, cone shaped to scoop shaped. Aphophysis short, alternate, each bearing a mamelon on dorsal side near node and supporting a hydrocladium. One or two branches, irregularly arranged, inserted at an angle of about 45º to the hydrocaulus, each replacing a hydrocladium and arising from an apophysis. Hydrocladia unbranched, up to 3.4 mm long, directed outward at an angle of about 60º from axis of hydrocaulus, divided into alternating athecate and thecate internodes each with two variably developed internal septa, one at each end; most proximal internode 90-330 µm long, with a straight node proximally and an oblique node distally, lacking hydrothecae and nematotheca; thecate internodes 238-409 µm long, each with an oblique node proximally, a straight node distally, a medium inferior nematotheca; athecate internodes beyond the most proximal one 192-590 µm long, with a straight node proximally, an oblique node distally, and one intermediate nematotheca. Nematotheca of hydrocladia bithalamic, movable, cone-shaped to scoop-shaped; median nematotheca 43-55 µm long, typically ending proximal to or just reaching the base of hydrotheca; lateral nematotheca 55-65 µm long, not reaching to margin of hydrotheca. Hydrotheca 148- 178 µm deep, cup-shaped, distal third or more of adcauline wall free from internode, axis oblique to that of internode; abcauline wall slightly convex basally, concave distally; margin entire, perpendicular or nearly so to axis of hydrothecae, aperture oval, in outline, diameter 115-144 µm; without intratecal septum. Cnidome comprising microbasic mastigophores 4.4-5.1 x 1.9-2.0 µm, pseudostenoteles 8.4-10,2 x 3.9-4.4 µm; and isorhizas 7.8-8.9 x 1.9-2.5 µm.
Known range: Bermuda (Calder 1983, 1993, 1997a); western Atlantic (Calder 1983); eastern Atlantic (Billard 1927); Indian Ocean (Millard 1975); western Pacific (Hirohito 1974); eastern Pacific, Point Vicente, California (Fraser 1948).
Family Halopterididae Millard, 1962
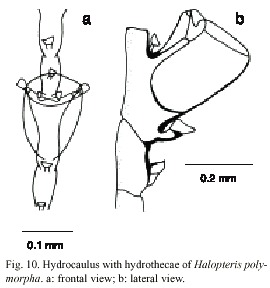
Halopteris polymorpha (Billard, 1913)
(Fig. 10)
Plumularia polymorpha Billard, 1913; Halopteris polymorpha Vervoort, 1966; Millard and Bouillon, 1973; Millard, 1975, Hirohito, 1983; Migotto, 1993; Schuchert, 1997.
Material examined: fourteen colonies (gonothecae not observed), Manzanillo, Limón, Caribbean coast, 9º3755 N, 82º3910 W, 19 September 1992 (UCR-691).
Description: colonies up to 32 mm, arising from a creeping hydrorhiza. Hydrocaulus monosiphonic, unbranched, base not segmented, lacking hydrocladia, with a variable number of nematothecae, separated by an oblique articulation; hydrocaulus divided at regular intervals by distinct transverse nodes; each caulinar internode with one hydrothecae, one apophysis laterally disposed and five to seven nematothecae (one mesial, two lateral, and two to four superior). Hydrocladia alternate, composed of one or two basal joints, followed by two athecate internodes with one nematotheca and alternately athecate. Athecate internode usually continued with the thecate one, with an inconspicuous node, or lacking a node. Hydrothecal internode with one hydrotheca and three or five nematothecae (one mesial and two laterally disposed), and with one or two reduced sessile nematothecae between hydrothecae and hydrocaulus. Hydrothecal margin 180-200 µm, and 60-128 µm deep; lateral nematothecae 76-128 µm long, placed on the apophysis. Cnidome comprising pseudostenoteles 18-22 x 7.6-9.2 mµm and microbasic mastigophores 6.0-7.1 x 1.8-2.1 µm.
Known range: Indian Ocean, Pacific (Billard 1913, Vervoort 1966, Millard and Bouillon 1973, Millard 1975, Hirohito 1983); Brazil (Migotto 1993).
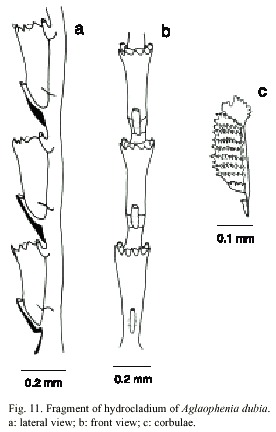
Aglaophaenia dubia Nutting, 1900
(Fig. 11)
Aglaophenia gracilis Allman, 1877 [Not Aglaophenia gracilis Lamouroux, 1816]; Aglaophenia dubia Nutting, 1900; Aglaophenia flowersi Nutting, 1900; Aglaophenia lophocarpa Bennit, 1922 [Not Aglaophenia lophocarpa Allman, 1877]; Aglaophenia allmani Leloup, 1935 [Not Macrorhynchia allmani (Nutting, 1900)]; Aglaophenia elongata Leloup, 1937; Deevey, 1954; van Gemerden-Hoogeven, 1965; Vervoort, 1968; Defenbaugh, 1974 [Not A.elongata Meneghini, 1845]; ?Aglaophenia dubia Fraser, 1938; Aglaophenia acacia Svoboda, 1979; Svoboda and Cornelius, 1991 [Not A.acacia Allman, 1883]; Aglaophenia apocarpa Calder, 1993 [Not Aglaophenia apocarpa Allman, 1877]; Aglaophenia sp. Calder, 1993; Aglaophenia dubia Calder, 1997a.
Material examined: eight colonies, Punta Pitahaya, Guanacaste, Pacific coast, 10º0355 N, 85º4557 W, 15 June 1991 (UCR-577).
Description: colonies up to 18 cm high, with a creeping hydrohiza. Hydrocaulus monosiphonic, wiry and slender, reaching 0.6mm wide basally; straight and unbranched in small colonies, gradually curved and branched in larger ones; basis of hydrocaulus devoid of apophysis, hydrocladia, nematophores, and regular nodes, merging imperceptibly and without an oblique hinge-joint with hydrocladium and nematophore-bearing part above; perisarc thick; nodes obliterated; cauline internodes marked by distinct to indistinct transverse nodes; each internode with a fronto-laterally situated hydrocladial apophysis, a pair of axillary nematothecae, and one or two inferior nematothecae. Apophysis short, given off alternately from two sides of hydrocaulus; each apophysis with a cone-shaped mamelon and supporting a hydrocladium. Cauline nematothecae scoop to sac-shaped; aperture single, keyhole-shaped. Branches originating from front of hydrocaulus, typically given off singly, unbranched or rebranched; anterior surfaces of branches generally facing hydrocaulus or branch from which they originate. Hydrocladia unbranched, about 18mm long, directed outward at an angle of about 50º from axis of hydrocaulus; each hydrocladium arising directly from an apophysis and divided by nearly transverse nodes into internodes; nodes sometimes indistinct. Hydrocladial internodes 335-490 µm long, 72-126 µm wide at nodes, each one with one hydrotheca placed frontally, one median inferior nematotheca and one pair laterally placed nematothecae. Two internodal septa, one placed laterally at the basis of intrathecal septum and other at basis of lateral nematothecae. Hydrothecae deeply cone-shaped, axis slighty oblique to that of internode; adcauline wall slightly convex, adnate to hydrocladial internode; abcauline wall basally convex, slightly concave distally from median to inferior nematothecal margin; intrathecal septum short; perisarc quite thin; abcauline wall lacking carina. Hydrothecal aperture 125- 208 µm in diameter, outline nearly round. Hydrothecal margin with nine acute cusps separated by U-shaped incisions. Lateral nematotheca duct-shaped, distal and bent forward and somewhat outward, terminal orifice scoop-shaped, reaching to or just beyond hydrothecal margin; base of each lateral nematothecae with an aperture into internode. Gonophores fixed sporosacs. Female corbula closed, up to 4 mm long, 1 mm wide, born on peduncles with from one to three cormidia; rachis straight to slightly curved, bearing alternate nematocladia indistinctly separated from metabasal cladia; either side of the rachis with six to twelve fused nematocladia, each with a row of hoof-shaped nematothecae. Cnidome comprising microbasic mastigophores of three sizes: (i) small, 4.5-5.4 x 1.6-2.0 µm; (ii) medium, 7.9-9.2 x 2.5-2.8 µm, and (iii) large, 26.4- 35.6 x 3.2-5.1 µm.
Known range: Bermuda (Bennitt 1922, Calder, 1993, 1997a); western Atlantic (Rees and Thursfield 1965); eastern Pacific (Fraser, 1938).
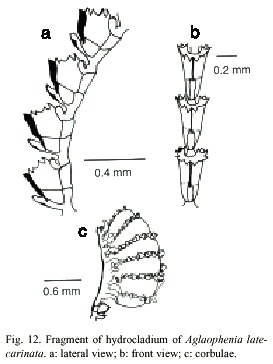
Aglaophenia latecarinata Allman, 1877
(Fig.12)
Sertularia pluma Bosc, 1802 [Not Aglaophenia pluma (Linnaeus, 1758)]. ?Aglaophenia pelagica Lamouroux, 1816 [Nomem dubium]. ?Plumularia simplex dOrbigny, 1846 [Nomem dubium].
?Aglaophenia simplex Kirchenpauer, 1872 [Not Aglaophenia perforata Kirchenpauer, 1876 (Nomem nudum)] [=Macrohynchia phillipina Kirchenpauer, 1872]. Aglaophenia perpusila Allman, 1877; Aglaophenia latecarinata Allman, 1877 [Incorrect original spelling]; Aglaophenia latecarinata Tizard et al. 1885 [Justified emendation]; Migotto, 1993; Calder, 1997a. Aglaophenia minuta Fewkes, 1881; Agalophenia perforata Allman, 1885; Aglaophenia mammilata Nutting, 1900; Aglaophenia minima Nutting, 1900; Anisocalyx (Aglaophenia) pelagica Bedot, 1905; Aglaophenia latecarinata madagascariensis Billard, 1907; glaophenoides mamillata Morris and Mogelberg, 1973; Not Aglaophenia latecarinata García et al, 1978; Aglaophenoides mammillatus Cairns et al, 1991 [Justified emendation].
Examined material: six colonies, with corbulae, Ocotal, Guanacaste, Pacific coast, 10°3254 N, 85°4330 W, 23 March 1984 (UCR-896).
Description: colonies up to 28 mm high, with a creeping, wrinkled hydrorhiza. Hydrocaulus monosiphonic, slender, unbranched, 0.1-0.2 mm wide basally, slightly convex frontally; perisarc thick basally, thinning progressively towards distal end. Basis of hydrocaulus with several irregular annulations just above the juncture with hydrorhiza. One or two large cauline nematothecae present; separated from hydrocladium-bearing part above by one short internode having a single nematothecae and oblique hinge-joints proximally and distally. Distal part of hydrocaulus divided into internodes by distinct to indistinct transverse nodes; each internode of hydrocaulus typically with a large, cone-shaped, inferior nematothecae, a fronto-laterally situated hydrocladial apophysis, and a pair of small, sac-shaped, axillary nematothecae; apophysis short, given off alternately from each side of hydrocaulus, adjacent pair on a given side about 0.60-1.0 mm apart; each apophysis with a cone shaped mamelon basally and supporting a hydrocladium distally; mamelom prominent, with a round to oval orifice. Hydrocladia reaching about 4 mm long, unbranched, directed outward at an angle of about 60º or more from the axis of hydrocaulus; frontal sides convex; top sides nearly straight; each hydrocladium arising directly from an apophysis and divided into short internodes by nearly transverse nodes. Hydrocladial internodes 215- 420 µm long, 50-60 µm wide at nodes, each one with one frontally placed hydrotheca, one median inferior nematotheca, and one pair of lateral nematothecae; internodal septa typically two, with one at base of intrathecal septum and another at the basis of lateral nematothecae, both well developed and reaching across internode, occasionally with an additional weakly developed septum between these two.
Hydrothecae close together, 241-318 µm deep from tip of median abcauline cusps to base, cone shaped, axis oblique to that of internode, adcauline wall convex, occasionally with a slight concavity at the proximal end, notched at the intrathecal septum, adnate to hydrocladial internode except for small free part at the distalmost end; abcauline wall convex basally, nearly straight to slightly concave from juncture of intrathecal septum to margin; prominent septum, slightly oblique, extending from adcauline to abcauline wall of hydrothecae; appearing in lateral view to divide cavity into two sections; abcauline wall with a carina of varying thickness, from thin and almost imperceptible to thick and keel-shaped, extending forward to mediam abcauline cusp, distal end of carina prow-shaped. Perisarc elsewhere of moderate thickness. Hydrothecal aperture 140- 168 µm in diameter from adcauline wall to base of median abcauline cusp, irregularly oval in outline, plane of orifice nearly perpendicular to long axis of the hydrothecae; margin with nine cusps all rounded and prominent, one median and four lateral pairs, separated by U-shaped incisions; cusps usually more or less equal in size. Median inferior nematothecae
anvil-shaped, adnate to abcauline wall of hydrothecae basally, small part free distally; apex reaching from one third to one half distance along abcauline wall of hydrothecae; distal operture scoop-shaped, or oval with an additional oval aperture at the juncture with abcauline wall of the hydrothecae; nematothecae directed outward, with terminal orifice scoop-shaped, reaching to or just beyond hydrothecal margin; base of each lateral nematothecae with an internodal aperture. Gonophores fixed sporosacs. Mature cobulae kidney-shaped up to 1.7 mm long, 1.1 mm wide, borne on peduncle having a single cormidium; rachis wrinkled, slightly curved, bearing alternate nematocladia arising from metabasal cladia; either side of the rachis with five to seven partially fused nematocladia each with one conspicuous anterior spur, small oval gaps laterally and distally on the corbulae between adjacent nematothecae. Cnidome comprising two sizes of microbasic mastigophores: (i) small, 4.9-5.6 x 1.8-2.2 µm, and (ii) large, 27-38 x 3.7-5.1 µm.
Known range: Bermuda (Congdon 1907, Smallwood 1910, Bennitt 1922, Morris and Mogelberg 1973, Butler et al. 1983, Calder 1986, 1993, 1995, 1997); western Atlantic (Vervoort 1968); eastern Atlantic (Vervoort 1968); Indian Ocean (Millard 1975); western Pacific (Hirohito 1983).
Macrorhynchia philippina Kirchenpauer, 1872; Rees and Vervoort, 1987; Calder, 1986; Migotto, 1993; Calder, 1997. Aglaophenia philippina Kirchenpauer, 1872; Aglaophenia urens Kirchenpauer, 1876 [Nomem Nudum]; Lytocarpus urens Bale, 1888; Aglaophenia (Lytocarpus) phillipina Bale, 1888 not Lytocarpus phillipinus Congdon, 1907 [=Macrorhynchia allmani Nutting, 1900, according to Calder (1997a)]; Lytocarpus crosslandi Ritchie, 1908; Lytocarpus philippinus atlanticus Billard, 1913; Aglaophenia (Macrorhynchia) philippina Stechow, 1919; Macrorhynchia crosslandi Stechow, 1923a; Macrorhynchia urens Stechow, 1923a; Lytocarpus (Macrorhynchia) philippinus Boero and Bouillon, 1987.
Examined material: twenty-two colonies with gonophores and phylactocarps, Conchal, Guanacaste, Pacific coast, 10º2355 N, 85º4845 W, 31 September 1976 (UCR- 614).
Description: colonies up to 9 cm high. Hydrocaulus arising from stolons. Hydrocaulus pinnately or twice pinnately branched, reaching up 1.3 mm in width basally. Hydrocaulus and branches polysiphonic except at distal ends, curved gradually backward; nodes inconspicuous. Cauline internodes each with one hydrocladial apophysis and two nematothecae, one distal axillary and one proximal; nematothecae sac-shaped, tapering to a slender cone distally, orifice small, spherical to oval. Hydrocladial apophysis short, each bearing a cone shaped mamelon on anterior surface; given off alternately from frontal surface of axial tubes of hydrocaulus and branches, quite closely spaced, directed outward at an angle of about 45º from axis of adjacent hydrocaulus or branch. Hydrocladia up to 5mm long, unbranched; top and frontal sides convex; each hydrocladium arising directly from apophysis and separated from it by an oblique node; divided into short internodes by nearly straight nodes. Hydrocladial internodes 240-305 µm long, 75-110 µm wide at nodes, each with one frontally placed hydrotheca, one median inferior nematotheca, one pair of lateral nematotheca, and two internodal septa; adjacent hydrothecae close to one another. Hydrothecae slipper-shaped, 250- 271 µm deep from adcauline wall at margin to base, curving abruptly outward distally; adcauline wall convex except for pronounced concavity just below margin; abcauline intrathecal septum conspicuous, cusp-shaped, curved away from hydrocaulus, extending about halfway across intrathecal cavity; hydrothecal aperture 130-156 µm in diameter from adcauline to abcauline wall, irregularly oval; plane of aperture oblique to axis of internodes and to that of hydrothecae; margin with an acute median abcauline cusp, a pair of low and rounded lateral cusps, and low and unconspicuous median adcauline cusp, and a flange on either side where rim meets lateral nematothecae; aperture distinctly flaring in top view. Median inferior nematothecae long, anvil-shaped, reaching beyond margin of hydrothecae distally, slightly curved, adnate to abcauline wall of hydrothecae as far as intrathecal septum, distal third free, cone-shaped, length free 118-139 mm; lateral nematothecae tubular, largely adnate to hydrothecal wall, distal end tapered and curved forward, extending a varying distance beyond margin of hydrothecae; terminal aperture small and spherical. Phylactocarps single, oval-shaped, slightly curved, up to 4.2 mm, unbranched; each with a basal thecate internode, followed by an internode with a gonotheca. Gonothecae as fixed small sporosac, oval-shaped and flattened laterally. Cnidome comprising microbasic mastigophores of three sizes: (i) small, 4.9-6.5 x 1.8-2.2 µm; (ii) medium, 20.0-30.0 x 3.9- 5.4 µm, and (iii) large, 95.0-100.0 x 7.1-7.9 µm.
Known range: Bermudas (Bennitt 1922, Calder 1983, 1986, 1993, 1997); western Atlantic (Vervoort 1968); eastern Atlantic (Ritchie 1908); Indian Ocean (Millard 1975); western Pacific (Hirohito 1983); eastern Pacific (Fraser 1948); Brazil (Migotto 1993).
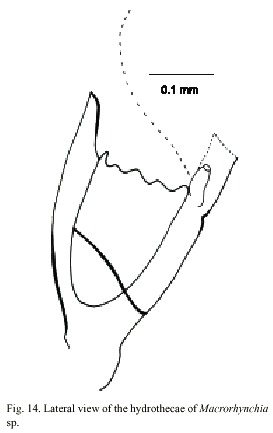
Macrorhynchia sp.
Fig. 14)
Macrorhynchia Kirchenpauer, 1872, Calder, 1997a. Nematophorus Clarke, 1879. Pleurocarpa Fewkes, 1881.
Examined material: nine damaged colonies, Punta Cabuja, Guanacaste, Pacific coast, 10°2220 N, 85°5125 W, 16 November 1991 (UCR-894).
Description: colony erect, up to 10 cm high; hydrocaulus branched, polysiphonic arising from anchoring filaments; cauline internodes with triangular nematothecae. Hydrocladia unbranched, arranged pinnately, arising alternately from apophyses on axial tube of hydrocaulus and branches. Hydrothecae 300-350 µm deep from tips of the seven marginal cusps to base, and usually cone-shaped; perisarc of moderate thickness; hydrothecal aperture 140-190 µm in diameter from adcauline to abcauline wall, irregularly oval. Each hydrotheca with a pair of lateral horn-shaped nematothecae and one partially adnate median inferior nematothecae. Gonophores not observed. Cnidome comprising microbasic mastigophores of three distinct sizes: (i) small, 4.6 x 1.4-2.0 µm, (ii) medium, 16.0-28.0 x 3.5-5.0 µm, and (iii) large, 90.0-95.0 x 6.5-7.5 µm.
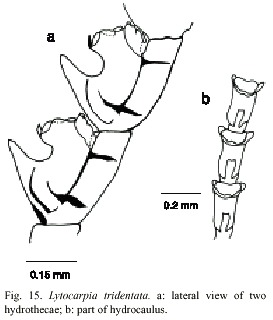
Lytocarpia tridentata (Verluys, 1899)
(Fig. 15)
Aglaophenia tridentata Verluys, 1899, Stechow, 1923b. Aglaophenia contorta Nutting, 1900; Bedot, 1921; Vannucci-Mendes, 1946; Vannucci, 1951. Thecocarpus contorta Totton, 1926, Aglaophenia tridentata Picard, 1951a; Vervoort, 1968; Forneris, 1969. Lytocarpia tridentata Migotto, 1993.
Examined material: nine colonies without gonophores, northern part of Isla Pájaros, Limón, Caribbean coast, 10°0110 N, 83°0445 W, 27 September 1996 (UCR-891).
Description: colonies up to 16 cm high, with a creeping hydrorhiza. Hydrocaulus monosiphonic and unbranched. Basal part of the colony usually without hydrocladia and nematothecae. Distal part divided into internodes by distinct transverse nodes. Internodes with one hydrocladial apophysis and four nematothecae: two axillary disposed, one inferior, and one frontal pseudonematothecae. Hydrocladia with distinct internodes, separated by oblique nodes. Each internode with one hydrotheca and three nematohecae: two laterally and one mesial. Hydrothecae with short internal septa, two almost imperceptible lateral teeth, and one conspicuos median tooth. Nine or eleven tentacles. Margin of hydrotheca about 166 mm in diameter and 280-336 µm deep. Mesial nematotheca up to 288 µm long; lateral nematothecae about 125 µm long. Perisarc of moderate thickness. Gonophores not observed. Cnidome comprising microbasic mastigophores of two categories: (i) small, 4.9-5.2 x 1.8-2.5 µm, and (ii) large, 28.0-36.0 x 4.1-5.2 µm.
Known range: western Atlantic (Vervoort 1968 as Aglaophenia tridentata); Brazil (Totton 1926, Vannucci-Mendes 1946, Vannucci 1951, Migotto 1993).
Family Campanulariidae Johnston, 1837
Subfamily Clytiinae Cockrell, 1911
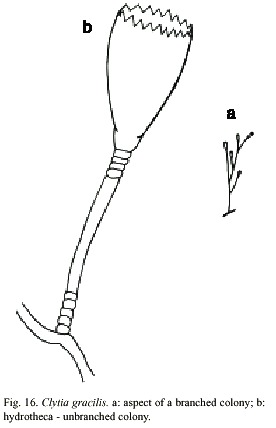
Clytia gracilis (Sars, 1850)
(Fig. 16)
Laomedea gracilis M. Sars, 1850; Laomedea (Campanularia) gracilis M. Sars, 1857; Clytia (Platypyxis) cilindrica L. Agassiz, 1862; Clytia cilindrica L. Agassiz, 1862; Platypyxis cylindrica L. Agassiz, 1862; Gonothyrea gracilis Allman, 1864; Campanularia gracilis Van Beneden, 1867; Clytia laevis Weismann, 1883; Campanularia serrulata Bale, 1888; Clytia serrulata Pictet, 1893; Laomedea (Gonothyraea) gracilis Levinsen, 1893; Campanularia attenuata Calkins, 1899; Obelia serrulata Thornely, 1900; Campanularia pelagica van Breemen, 1905; Clytia coronata Fraser, 1912; Clytia elsae-oswaldae Stechow, 1914; Clytia pelagica Billard, 1917; Clytia gracilis Stechow, 1923a; Calder, 1990. Laomedea (Phialidium) pelagica Hummelinck, 1930; Laomedea (Phialidium) gracilis Hummelinck, 1930; Laomedea pelagica Hummelinck, 1930; Laomedea (Clytia) gracilis Broch, 1933; Laomedea gracilis form pelagica Leloup, 1933; Clytia (campanularia) pelagica Kunne, 1937; Laomedea cylindrica Leloup, 1937; Clytia hemisphaerica Millard, 1966; Cornelius, 1982. Clytia elsaeoswaldea Vervoort, 1968; Campanularia (Clytia) cylindrica Vervoort, 1968; Laomedea (Clytia) pelagica Vervoort, 1972; Clytia sarsi Cornelius, 1982.
Material examined: twenty three colonies with gonothecae, Isla del Coco, Pacific coast, 5°2981 N, 87°0309 W, 17 June 1994 (UCR-832).
Description: colonies erect, fragile, sparingly and irregularly branched, up to 10 mm high, always arising from a creeping hydrorhiza. Hydrocaulus monosiphonic; each branch arising from a slightly curved aphophysis given off below hydrothecae of pedicel from which it arises; branches resembling primary pedicels and directed abruptly upwards, 6-10 annulations proximally and 2-6 distally placed. Pedicels about 0.5-0.4 mm high, 101- 142 µm in diameter, annulated basally and distally, with annuli or wrinkles elsewhere. Perisarc firm. Hydrothecae more or less cone-shaped, about 730-930 µm long, 390 µm wide at margin, 165-244 µm wide at diaphragm, with thin perisarc. Walls convex above diaphragm, nearly straight elsewhere; margin with about 12 to 17 deeply cut, blunt, triangular tooth cylindric by U-shaped incisions; hydrothecal walls weakly scalloped in cross section at margin. Diaphragm thin, straight; the basal chamber varied in depth but usually deep and cup-shaped. Tentacles small, 18-21 in number, but usually 20. Gonothecae urn-shaped, about 968-1000 µm long, and 404-432 mm in diameter; orifice diameter about 305- 342 µm wide, constricted just below aperture to about 320 µm wide, arising from hydrorhiza on short pedicels; walls smooth, constricted below aperture. Cnidome comprising ishorhizas about 5.8-6.5 x 1.6-1.9 µm and microbasic mastigophores of three distinct sizes; (i) small, 6.5-7.8 x 1.8-2.6 µm; (ii) medium, 8.4-9.2 x 2.8-3.1 µm; and (iii) large,13- 16.3 x 3.1-3.9 µm.
Known range: Bermudas (Bennitt 1922 and Calder 1986 as Clytia Cylindrical, Calder 1990); western Atlantic (Vervoort 1968 as Laomedea (Phialidium) pelagica); eastern Atlantic (Vervoort 1946 as Laomedea pelagica); Indian Ocean (Mammen 1965); western Pacific (Yamada 1959); eastern Pacific (Fraser 1948 as Gonothyrea gracilis); Brazil (Migotto 1993).
Discussion
The most recent report on the fauna of hydroids from Costa Rica (Cortés 1997), provided a list of 69 species of hydrozoans, including seven orders: Siphonophora, Stylasterina, Anthoathecatae, Leptothecatae, Leptomedusae, Narcomedusae and Trachymedusae. In this study, we introduce 16 new records to the list (Table 1), increasing the total number of species of the class Hydrozoa from Costa Rica to 75.
The list presented by Cortés (1997) includes 20 species of both Anthoathecatae and Leptothecatae orders. However, the reviewed synonym reduced this number to 19 (Table 2). With the additional contribution from this study, the actual number of Anthoathecate and Leptothecate species appears to be 35, of which only 16 are stored at the Museum of Zoology, University of Costa Rica.
The species Halopteris polymorpha (Billard 1913) previously reported in the Indian and Pacific Oceans (Billard 1913, Vervoort 1966, Millard and Bouillon 1973, Millard 1975, Hirohito 1983) and Brazil (Migotto 1993), is reported for first time from Caribbean Sea.
Another two species are new additions to the eastern Pacific fauna: Acryptolaria longitheca (Allman 1877), previously known from western Atlantic and Australia (Allman 1877, 1888, Clark 1879, Vervoort 1972, Calder 1996, Calder and Vervoort 1998), and Agloaphenia latecarinata Allman, 1877, from Bermuda (Congdon 1907, Smallwood 1910, Benitt 1922, Moris and Mogelberg 1973, Butler et al. 1983, Calder 1986, 1993, 1995, 1997a), western and eastern Atlantic (Vervoort 1968), Indian Ocean (Millard 1975), western Pacific (Hirohito 1983). Finally, the southerly range of Plumularia floridana Nutting, 1900, is now extended from Vicente Point, California to Punta Pitahaya, Guanacaste.
The Anthoathecatae and Leptothecatae hydroids are a diverse and conspicuous group of marine invertebrates. Most of the species have an apparent cosmopolitan distribution, with records from almost all oceans. However, the number of species reported from Costa Rica is still comparatively low, and unfortunately, the degree of preservation of the voucher material restricted the observation of any morphological variation. Additionally, although the hydroids are able to produce phenotypic responses to environmental changes (Kelmo and Attrill, in press), the absence of quantitative ecological data impedes the formulation of any robust conclusion about the distribution or ecology of the group. Therefore, there is a requirement for further baseline research on this group for both Pacific and Caribbean coasts of Costa Rica.
Acknowledgements
This work was made possible by financial support from CNPq-Brazil and Centro de Apoio ao Desenvolvimento Científico e Tecnológico - Secretaria de Planejamento e Tecnologia do Estado da Bahia (CADCT-SEPLANTEC). The authors wish to thank to Dale Calder from Centre for Biodiversity and Conservation Biology of Royal Ontario Museum (Canada) for critical comment of manuscript and to J. Cortés (Escuela de Biología y Centro de Investigación en Ciencias del Mar y Limnología, CIMAR), Universidad de Costa Rica for the collection of the specimens. Gratitude is expanded to Simone Souza de Moraes, Rilza da Costa Tourinho Gomes and Larissa de Siqueira, for all encouragement provided. This is a contribution of Museo de Zoología, Escuela de Biología, Universidad de Costa Rica.
Resumen
Este estudio proporciona el primer informe taxonómico de los hidroides de los Ordenes Anthoathecatae y Leptothecatae presentes en las colecciones del Museo de Zoología de la Escuela de Biología de la Universidad de Costa Rica. Se informan 16 especies de hidroides: Eudendrium carneum, Pennaria disticha, Acryptolaria longitheca, Plumularia floridana, Halopteris polymorpha, Aglaophenia dubia, Aglaophenia latecarinata, Lytocarpia tridentata, Macrorhynchia phillipina, Macrorhynchia sp., Clytia gracilis, Cnidoscyphus marginatus, Thyroscyphus ramosus, Dynamena disticha, Sertularella diaphana y Tridentata distans. Se presenta una exhaustiva revisión de la sinonimia y una clave taxonómica simple e ilustraciones y descripciones para cada una de las especies.
References
Agassiz, A. 1865. North American Acalephae. Illustrated Catalogue of the Museum of Comparative Zoology. Harvard College, Cambridge No II, 234 p. [ Links ]
Agassiz, L. 1860. Contributions to the natural history of the United States of America. Vol III. Little Brown & Company, Boston. 301 p. [ Links ]
Agassiz, L. 1862. Contributions to the natural history of the United States of America. Vol. IV. Little Brown & Company, Boston. 380 p. [ Links ]
Allman, G.J. 1864. On the construction and limitation of genera among the Hydroida. Ann. Mag. Nat. Hist. 13: 345-380. [ Links ]
Allman, G.J. 1872. A monograph of the gymnoblastic or tubularian hydroids. Conclusion of the part I and part II. Roy. Society, London. pp. 155-450 [ Links ]
Allman, G.J. 1877. Report on the Hydroida collected during the exploration of the Gulf Stream by L. F. de Portualès, assistant United States Coast Survey. Mem. Mus. Comp. Zool. Harvard Coll. 5: 1-66. [ Links ]
Allman, G.J. 1883. Report on the Hydroida dredged by H.M.S. Challenger during the years1873-76. Part I. Plumularidae. Report on the Scientific Results of the Voyage of H.M.S. Challenger during the years 1873- 76. Zoology 7: 1-54. [ Links ]
Allman, G.J. 1885. Description of Australian, Cape, and other hydroida, mostly new, from the collection of Miss H.Gatty. J. Linn. Soc. Zool. 19: 132-160. [ Links ]
Allman, G.J. 1888. Report on the Hydroida dredged by H. M. S. Challenger during the years 1873-76. Part II: The Tubularinae, Corymorphinae, Campanularinae, Sertularinae, and Thalamophora. Report on the Scientific Results of the Voyage H. M. S. Challenger during the years 1873-76. Zoology 23: 1-90. [ Links ]
Altuna Prados, A. 1994. Estudio faunístico, ecológico y biogeográfico de los cnidarios bentónicos de la Costa Vasca. Ph.D. Dissertation. Universidad de Navarra. Pamplona, España. 769 p. [ Links ]
Altuna Prados, A. 1995. Observaciones geográficas sobre los cnidarios bentónicos de la Costa Vasca. Kobie 22: 41-57. [ Links ]
Ayres, W.O. 1854. Description of new species Polyps: Globiceps tiarella. Proc. Boston Soc. Nat. Hist. 4: 193-195. [ Links ]
Bale, W.M. 1884. Catalogue of Australian hydroid zoophytes. Australian Mus. 8: 1-198. [ Links ]
Bale, W.M. 1888. On some new and rare Hydroida in the Australian Museum Collection. Proc. Linn. Soc. New South Wales 2: 745-799. [ Links ]
Bale, W.M. 1894. Further notes on Australian hydroids, with descriptions of some new species. Proc. Roy. Soc. Victoria 16: 93-117. [ Links ]
Bale, W.M. 1913. Further notes on Australian hydroids II. Proc. Roy. Soc. Victoria 26: 114-147. [ Links ]
Bale, W.M. 1919. Further notes on Australian hydroids IV. Proc. Roy. Soc. Victoria 31: 327-361. [ Links ]
Bedot, M. 1905. Matériaux pour servir a lhistoire des hydroïdes. 2éme période (1821 à 1850). Rev. Suis. Zool. 13: 1-183. [ Links ]
Bedot, M. 1912. Matériaux pour servir a lhistoire des hydroïdes. 4éme période (1872 à 1880). Rev. Suis. Zool. 20: 213-469. [ Links ]
Bedot, M. 1916. Matériaux pour servir a lhistoire des hydroïdes. 5éme période (1881 à 1890). Rev. Suis. Zool. 24: 1-349. [ Links ]
Bedot, M. 1918. Matériaux pour servir a lhistoire des hydroïdes. 6e période (1891 à 1900). Rev. Suis. Zool. 24: 1-376. [ Links ]
Bedot, M. 1921. Hydroïdes provenant des campagnes des yachts Hirondelle et Princesse-Alice (1887 à 1912). I. Plumularidae. Résultats des Campagnes Scientifiques Accomplies sur son Yacht par Albert Iér, Prince Souverain de Monaco 60: 1-70. [ Links ]
Bedot, M. 1925. Matériaux pour servir a lhistoire des hydroïdes. 7éme période (1901 à 1910). Rev. Suis. Zool. 32 (Suppl.): 1-657. [ Links ]
Bennitt, R. 1922. Additions to the hydroid fauna of the Bermuda. Contributions from the Bermuda Biological Station for Research No 136. Proc. Amer. Acad. Arts Sci. 57: 241-259. [ Links ]
Billard, A. 1906. Note sur les hidroïdes du Travailler et du Talisman. Bull. Mus. Hist. Nat. Paris 12: 329-334. [ Links ]
Billard, A. 1907. Hydroïdes de Madagascar et du sudest de lAfrique. Arch. Zool. Exp. Gén. 7: 335-396. [ Links ]
Billard, A. 1910. Revision dune partie de la collection des hydroïdes du British Museum. Ann. Sci. Nat. Zool. 11: 1-67. [ Links ]
Billard, A. 1912. Hydroïdes de Roscoff. Arch. Zool. Exp. Gén. 51: 459-478. [ Links ]
Billard, A. 1913. Les hydroïdes de lexpédition du Siboga. I. Plumulariidae. Siboga-Expeditie, Monographie 7a: 1-115. [ Links ]
Billard, A. 1917. Note sur quelques espèces dhydroïdes libres. Bull. Mus. Nat. Hist. Nat. Paris 23: 539-546. [ Links ]
Billard, A. 1919. Notes sur quelques espèces nouvelles de Sertularella de lExpédition du "Siboga". Arch. Zool. Exp. Gén. 58: 18-23. [ Links ]
Billard, A. 1921. Note sur une variété de Sertularella (Sertularella diaphana madagacariensis). Bull. Mus. Nat. Hist. Nat. Paris 27: 184-186. [ Links ]
Billard, A. 1925. Les hydroïdes de lexpédition du Siboga II. Syntheciidae et Sertulariidae. Siboga-Expeditie. Monographie 7b: 115-232. [ Links ]
Billard, A. 1927. Les hydroïdes de la côte atlantique de France. Compt Rend. Congr. Soc. Savantes Paris, Séc. Sci 1926: 326-346. [ Links ]
Blainville, H.M.D. 1830. Zoophytes, Zoophyta. In F.G. Levrault (ed.). Dictionnaire des sciences naturelles. Tome 60. Le Normant, Paris. 548 p. [ Links ]
Blanco, O.M. 1994a. Claves de familias y géneros para facilitar el reconocimiento de los hydroids (Leptolina) Athecata, Thecata y Limnomedusae Argentinos (generación polipoide exclusivamente). Rev. Mus. Plata (Zool) 14(160): 147-179. [ Links ]
Blanco, O.M. 1994b. Enumeración sistemática y distribuición geográfica preliminar de los hydroida de la República Argentina, Suborden Athecata (Gymnoblastea, Anthomedusae), Thecata (Calyptoblastea, Leptomedusae) y Limnomedusae. Rev. Mus. Plata (Zool) 14(161): 181-216. [ Links ]
Boero, F. & J. Bouillon. 1987. Inconsistent evolution and paedomorphosis among the hydroids and medusae of the Athecatae/Anthomedusae and the Thecatae/Leptomedusae (Cnidaria, Hydrozoa) p. 229-250. In J. Bouillon, F. Boero, F. Cicogna & P. F. S. Cornelius (eds.). Modern trends in the systematics, ecology, and evolution of hydroids and hydromedusae. Claredon, Oxford. [ Links ]
Bonnevie, K. 1899. Hydroida. Den Norske Nordhavs-expedition 1876-1878, Zoologi, 7. Christiania, GD ndahal and SD n. 103 p. [ Links ]
Boone, L. 1938. Scientific results of the world cruises of the yatchs "Ava", in 1928-1929, and "Alva", 1931- 1932, "Alva" Mediterranean cruise, 1933, and "Alva" South America cruise, 1935, William K. Vanderbilt, Commanding. Part II: Coelenterata. Bulletin of the Vanderbilt Mar. Mus. 7: 29-76. [ Links ]
Bosc, L.A.G. 1802. Histoire naturelle des vers, contenant leur description et leurs moeurs; avec figures dessinées daprès nature. Tome 3. Guilleminet, Paris. 270 p. [ Links ]
Bouillon, J. 1985. Essai de classification des hydropolypes-hydroméduses (Hydrozoa-Cnidaria). Indo-Malayan Zool. 1: 29-243. [ Links ]
Bouillon, J., C. Massin & R. Kresevic. 1995. Hydromedusae de lInstitut Royal des Sciences Naturelles de Belgique. Doc. Trav. I.R.Sc.N.B. 78: 3- 106. [ Links ]
Brinckmann-Voss, A. 1970. Anthomedusae/Athecata (Hydrozoa, Cnidaria) of the Mediterranean, part I. Capitata. Fauna e Flora del Golfo di Napoli 39: 1-96. [ Links ]
Broch, H. 1933. Zur Kenntnis des Adriatischen Hydroidenfauna von Split. Arten und variationem. Skrifter Utgitt av det Norske Videnskaps-Akademi i Oslo I. Mat.-Natur. Kl. 1933: 1-115. [ Links ]
Brooks, W.K. 1883. Notes on the medusae of Beaufort, N.C. Part II. Stud. Biol. Lab. Johns Hopkins Univ. 2: 465-475. [ Links ]
Browne, E.T. 1907. The hydroids collected by the Huxley from the north side of the Bay of Biscay in August 1906. J. Mar. Biol. Asso. U. K. 8: 15-36. [ Links ]
Busk, G. 1852. An account of the Polyzoa, and sertularian zoophytes, collected in the voyage of the "Rattlesnake", on the coasts of Australia and the Louisiade Archipelago. p. 343-402. In J. MacGillivray (ed.). Narrative of the voyage of H.M.S. Rattlesnake, commanded by the late Captain Owen Stanley, R.N., F.R.S., during the years 1846- 1850. Vol.1, Append. 4. T. & W. Boone, London. [ Links ]
Butler, J.N., B.F. Morris, J. Cadwallader & A.W. Stoner. 1983. Studies of Sargassum and the Sargassum community. Bermuda Biol. Sta. (Spec. Publ.) 22: 1-306. [ Links ]
Cairns, S.D.; D.R. Calder; A. Brinckmann-Voss; C.B. Castro; P.R. Pugh; C.E. Cutress; W.C. Jaap; D.G. Fautin; R.J. Larson; G.R. Harbison; M.N. Arai & D.M. Opresko. 1991. Commom and scientific names of aquatic invertebrates from the United States and Canada: Cnidaria and Ctenophora. Amer. Fish. Soc. (Spec. Publ.) 22: 1-75. [ Links ]
Calder, D.R. 1983. Hydroida from estuaries of South Carolina, USA: families Sertulariidae and Plumulariidae. Proc. Biol. Soc. Wash. 96: 7-28. [ Links ]
Calder, D.R. 1986. Class Hydrozoa. p. 127-155. In W. Sterrer (ed.). Marine fauna and flora of Bermuda: a systematic guide to the identification of marine organisms. Wiley-Interscience, New York. [ Links ]
Calder, D.R. 1988. Shallow water hydroids of Bermuda: the Athecatae. Roy. Ontario Mus., Life Sci. Contrib. 148: 1-107. [ Links ]
Calder, D.R. 1990. Seasonal cycles of activity and inactivity in some hydroids from Virginia and South Carolina, USA. Can. J. Zool. 68: 442-450. [ Links ]
Calder, D.R. 1991. Shallow water hydroids from Bermuda: the Thecatae, exclusive of Plumularioidea. Roy. Ontario Mus., Life Sci. Contrib. 154. 140 p. [ Links ]
Calder, D.R. 1993. Local distribution and biogeography of the hydroids (Cnidaria) of Bermuda. Carib. J. Sci. 29: 61-74. [ Links ]
Calder, D.R. 1996. Hydroids (Cnidaria: Hydrozoa) recorded from depths exceeding 3000 m in the western North Atlantic. Can. J. Zool. 74: 1721-1726 [ Links ]
Calder, D.R. 1997a. Shallow water hydroids of Bermuda: superfamily Plumularioidea. Roy. Ontario Mus., Life Sci. Contrib. 161: 1-85. [ Links ]
Calder, D.R. 1997b. Synopsis of hydroids from 1000 m and deeper in the western North Atlantic. p. 85-90. In J.C. Denhartog et al. (eds.). Proc. 6th Int. Conf. Coelenterate Biol. Leiden. The Netherlands. [ Links ]
Calder, D.R. & W. Vervoort. 1998. Some hydroids (Cnidaria: Hydrozoa) from the Mid-Atlantic Ridge, in the North Atlantic Ocean. Zool. Verhandel. 319: 1-65. [ Links ]
Calkins, G.N. 1899. Some hydroids from Puget Sound. Proc. Boston Soc. Nat. Hist. 28: 333-368. [ Links ]
Cavolini, F. 1785. Memorie per servire alla storia depolipi marini. Napoli. 279 p. [ Links ]
Clarke, S.F. 1879. Report on the hydroida collected during the exploration of the Gulf Stream and Gulf of Mexico by Alexander Agassiz, 1877-78. Bull. Mus. Comp. Zool. Harvard Coll. 5: 239-252. [ Links ]
Clarke, S.F. 1882. New and interesting hydroids from Chesapeake Bay. Mem. Boston Soc. Nat. Hist. 3: 135-142. [ Links ]
Clarke, S.F. 1907. Reports on the scientific results of the expedition to eastern tropical Pacific, in charge of Alexander Agassiz, by the U.S. Fish Comission steamer "Albatross", from october, 1904, to March, 1905, Lieut.–Commander L.M. Garret, U.S.N., commanding. VIII. The hydroids. Mem. Mus. Comp. Zool. Harvard Coll. 35: 1-18. [ Links ]
Cockrell, T.D.A. 1911. The nomenclature of the hydromedusae. Proc. Biol. Soc. Wash. 24: 77-86. [ Links ]
Congdon, E.D. 1906. notes on the morphology and development of two species of Eudendrium. Biol. Bull. 11: 27-46. [ Links ]
Congdon, E.D. 1907. The hydroids of Bermuda. Proc. Amer. Acad. Arts Sci. 42: 463-485. [ Links ]
Cornelius, P.F.S. 1979 A revision of the species of Sertulariidae (Coelenterata: Hydroida) recorded from Britain and nearby seas. Bull. Brit. Mus. Nat. Hist. Zool. 34: 243-321. [ Links ]
Cornelius, P.F.S. 1982. Hydroids and medusae of the family Campanularidae recorded from the eastern North Atlantic, with a world synopsis of genera. Bull.Brit. Mus. Nat. Hist. Zool. 42: 37-148. [ Links ]
Cornelius, P.F.S. 1992. The Azores hydroid fauna and its origin, with discussion of rafting and medusa supression. Arquipélago 10: 75-99. [ Links ]
Cortés, J. 1997. Biodiversidad marina de Costa Rica: Filo Cnidaria. Rev. Biol. Trop. 44(3)/45(1): 323-334. [ Links ]
Costa, O.G. 1842. Fauna del regno di Napoli. Zoofiti. Genere Anisocalice; Anysocalix, Don. Napoli, Azzolino e Compagno. 24 p. [ Links ]
Cowden, R.R. 1964. A cytochemical study of gonophore and oocyte developement in Pennaria tiarella. Acta Embryol. Morphol. Exp. 7: 167-179. [ Links ]
Cowden, R.R. 1965a. A cytological and cytochemical study of hydranths of the hydroid coelenterate, Pennaria tiarella. Zeitsch. Zellforsch. Mikroskop. Anat. 65: 869-883. [ Links ]
Cowden, R.R. 1965b. Cytochemical studies of embryonic development to metamorphosis in the gymnoblastic hydroid, Pennaria tiarella. Acta Embryol. Morphol. Exp. 8: 221-231. [ Links ]
Deevey, E.S. 1954. Hydroids of the Gulf of Mexico. Fish. Bull. 55: 267-272. [ Links ]
Defenbaugh, R. 1974. Hydroids. 94-112. In T.J. Bright & L.H. Pequegnat (eds.). Biota of the West Flower Garden Bank. Gulf Publ. Co., Houston. [ Links ]
Deshayes, G.P. & H. Milne-Edwards. 1836. Histoire naturelle des animaux sans vertèbres par J.B.P.A. de Lamarck. 2 éd. Tome 2. Baillière, Paris. 683 p. [ Links ]
Driesch, H. 1889. Tektonische Studien au Hydropolypen. Jena. Z. Naturw. 24(1): 189-226. [ Links ]
Ehrenberg, C.G. 1834. Beiträge zur physiologischen Kenntniss der Corallenthiere im allgemeinen, und besonders des Rothen Meers, nebst einem Versuche zur physiologischen Systematik derselben. Abhandl. Königlich. Akad. Wiss. 1: 225-380. [ Links ]
Fewkes, J.W. 1881. Reports on the results of dredging, under the supervision of Alexander Agassiz, in the Caribbean Sea, in 1878, 1879, and along the Atlantic coast of the United States, during the summer of 1880, by the US Coast Survey Steamer "Blake", Commander J.R.Bartlett, USN, commanding. XI. Report on the Acalephae. Bull. Mus. Comp. Zool. Harvard Coll. 8: 127-140. [ Links ]
Forneris, L. 1969. Fauna bentônica da baía do Flamengo, Ubatuba. Tese de Livre Docência (Thesis), Faculdade de Filosofia, Ciências e Letras, Universidade de São Paulo. São Paulo. 215 p. [ Links ]
Fraser, C.M. 1912. Some hydroids of Beaufort, North Carolina. Bull. U. S. Bur. Fish. 30: 339-387. [ Links ]
Fraser, C.M. 1937. Hydroids of the Pacific coast of Canada and the United States. University of Toronto, Toronto. 207 p. [ Links ]
Fraser, C.M. 1938. Hydroids of the 1932-38 Allan Hancock Pacific Expedition. Allan Hancock Pac. Exped. 4: 1-153. [ Links ]
Fraser, C.M. 1943. Distribution records of some hydroids in the collection of the Museum of Comparative Zoology at Harvard College, with description of new genera and new species. Proc. New Engl. Zool. Club 22: 75-98. [ Links ]
Fraser, C.M. 1944. Hydroids of the Atlantic coast of North America. University of Toronto, Toronto. 451 p. [ Links ]
Fraser, C.M. 1948. Hydroids of the Allan Hancock Pacific Expeditions since March, 1938. Allan Hancock Pac. Exped. 4: 179-335. [ Links ]
García, P., A. Aguirre & D. Gonzalez. 1978. Contribuición al conocimiento de los hidrozoos de las costas Españolas. Parte I: Halecidos, campanularidos y plumularidos. Bol. Inst. Españ. Oceanogr. 4: 3-73. [ Links ]
García, P., A. Aguirre & D. Gonzalez. 1980. Contribución al conocimiento de los hidrozoos de las costas Españolas. Parte III. "Serulariidae" Bol. Inst. Españ. Oceanogr. 6: 5-67. [ Links ]
García-Corrales, P. & A. Aguirre. 1985. La especie Halocordyle disticha (Goldfuss, 1820), y sus sinonimias. Bol. Inst. Españ. Oceanogr. 2: 85-96. [ Links ]
Golfuss, G.A. 1820. Handbuch der Zoologie I. Abtheilung. Nürnberg, Johan Leonhard Schrag. 696 p. [ Links ]
Gravier-Bonnet, N. 1979. Hydraires semi-profonds de Madasgacar (Coelenterata Hydrozoa), étude systématique et écologique. Zool. Verhandel. 169: 1-76. [ Links ]
Haeckel, E. 1879. Das System der Medusen. Erster Theil einer Monographie der medusen. Denkschriften der Medizinisch-Naturwissenschaftlichen Gesellschaft zu Jena, 1. 360 p. [ Links ]
Hargitt, C.W. 1924. Hydroids of the Philippine Islands. Philippine J. Sci. 24: 467-507. [ Links ]
Hartlaub, C. 1901. Revision der Sertularella-Arten. Abhandl. Geb. Natur. 16: 1-143. [ Links ]
Hassall, A.H. 1848. Definitions of three new British zoophytes. The Zoologist 6: 2223. [ Links ]
Hertlein, L.G. 1963. Contribution to the biogeography of Cocos Island, icluding a bibliography. Proc. Calif. Acad. Sci. 32: 219-289. [ Links ]
Hirohito. 1969. Some hydroids of the Amakusa Islands. Biological Laboratory, Imperial Household, Tokyo. 32 p. [ Links ]
Hirohito. 1974. Some hydrozoans of the Bonin Islands. Biological Laboratory, Imperial Household, Tokyo. 55 p. [ Links ]
Hirohito. 1983. Hydroids from Izu Ôshima and Niijima. Biological Laboratory, Imperial Household, Tokyo. 83 p. [ Links ]
Hummelinck, P.W. 1930. Beiträge zur Kenntnis Holländischer Hydroiden I. Bemerkungen über einige Camapnuliden und Camanulariden vom Vagdam und Nieuwediep. Tijdschr. Ned. Dierkund. Ver. 2: 28-42. [ Links ]
Jäderholm, E. 1907. Über einige nordische Hydroiden. Zool. Anz. 32: 371-376. [ Links ]
Jäderholm, E. 1919. Zur Kenntnis der Hydroidenfauna Japans. Arkh. Zool. 12: 1-34. [ Links ]
Jäderholm, E. 1920. On some exotic hydroids in the Swedish Zoological State Museum. Arkh. Zool. 13: 1-11. [ Links ]
Jarvis, F.E. 1922. The hydroids from the Chagos, Seychelles and other islands and from coasts of British East Africa and Zanzibar. Trans. Linn. Soc. London Zool. 18: 331-360. [ Links ]
Johnston, G. 1837. A catalogue of the zoophytes of Berwickshire. History of Berwickshire Naturalists Club 1: 107-108. [ Links ]
Kelmo, F. & L.M. Santa-Isabel. 1998. Athecatae hydroids (Cnidaria-Hydrozoa) from Northern Bahia, Brazil. Proc. 280 Meet. Asso. Mar. Lab. Caribbean. Rev. Biol. Trop. 46(Suppl. 5): 61-72. [ Links ]
Kelmo F. & M. Attrill. (in press). Cnidaria community structure of coastal reefs from northern Bahia, Brazil. Bull. Mar. Sci. [ Links ]
Kirchenpauer, G.H. 1872. Ueber die Hydroidenfamilie Plumularidae, einzelne Gruppen derselben und ihre Fruchtbehälter. I. Aglaophenia Lx. Abhandl. Geb. Natur. Herausg. Naturwiss. Ver. Hamburg-Altona 5: 1-52. [ Links ]
Kirchenpauer, G.H. 1876. Ueber die Hydroidenfamilie Plumularidae, einzelne Gruppen derselben und ihre Fruchtbehälter. II. Abhandl. Geb. Natur. Herausg. Naturwiss. Ver. Hamburg-Altona 6: 1-59. [ Links ]
Kirkpatrick, R. 1910. Hydrozoa and Porifera. Proc. Zool. Soc. London 1910: 86-131. [ Links ]
Künne, C. 1937. Über die Verbreitung der leitformen des Grossplanktons in der südlichen Nordsee in winter. Ver. Deut. Wiss. Komm. Meer. 8: 131-164. [ Links ]
Lamouroux, J.V.F. 1812. Extrait dun memoire sur la classification des polypieres coralligènes non entièrement pierreux. Nouv. Bull. Sci. Soc. Philom. Paris 3: 181-188. [ Links ]
Lamouroux, J.V.F. 1816. Histoire des polypieres coraligènes flexibiles, vulgairement nommés zoophytes. F. Poisson, Caen. 559 p. [ Links ]
Leidy, J. 1855. Contributions towards a knowledge of the marine invertebrate fauna, of the coasts of Rhode Island and New Jersey. J. Acad. Nat.l Sci. Philadelphia, 2nd series, 3: 135-152. [ Links ]
Leloup, E. 1932. Lhomologie des parties constituantes du gonossome chez Thecocarpus et Aglaophenia et la classification des Aglaopheniidae. Bull. Mus. Roy. Hist. Nat. Belg. 8: 1-26. [ Links ]
Leloup, E. 1933. Contribution à la conaissance des hydropolypes dela côte des Pays-Bas. Bull. Mus. Roy. Hist. Nat. Belg. 9 (45): 1-30. [ Links ]
Leloup, E. 1935. Hydraires calyptoblastiques des indes Ovidentales. Mém. Mus. Roy. Hist. Nat. Belg. 2: 1-73. [ Links ]
Leloup, E. 1937. Résultats scientifiques des croisières du navire-école belge "Mercator". VI. Hydroidea, Siphonophora, Ceriantharia. Mém. Mus. Roy. Hist. Nat. Belg. 9: 91-121. [ Links ]
Leloup, E. 1940. Hydropolypes provenant des croisières du Prince Albert I de Monaco. Résultats des Campagnes Scientifiques Accomplies sur son Yacht par Albert I, Prince Souverain de Monaco, Fasc. 104. 39 p. [ Links ]
Leloup, E. 1974. Hydropolypes calyptoblastiques du Chili. Report nº48 of the Lund University Chile Expedition 1948-1949. Sarsia 55: 1-61. [ Links ]
Lesh-Laurie, G.E. 1976. Stolon vs hydranth determination in Pennaria tiarella planulae: a role for DNA synthesis. p. 365-375. In G.O. Mackie (ed.). Coelenterata Ecology Behavior. Plenum Press, New York. [ Links ]
Levisen, G.M.R. 1893. Annulata, Hydroidae, Anthozoa, Porifera. Det Videnskabelige udbytte af Kanonbaaden "Hauchs" Togter i de Danske have indenfor Skagen i aarene 1883-86. Kjøbenhavn, Andr. Fred. Høst & Søns. pp. 317-427. [ Links ]
Linnaeus, C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species cum characteribus, differentis, synonymis, locis. 10 ed. reformata. Holmiae, Laurentii Salvi. 823 p. [ Links ]
Mammen, T.A. 1963 On a collection of hydroids from south India I. Suborder Athecata. J. Mar. Biol. Asso. India 5: 27-61. [ Links ]
Mammen, T.A. 1965. On a collection of hydroids from south India. III. Family Plumulariidae. J. Mar. Biol. Ass. India 7: 291-324. [ Links ]
Marktanner-Turneretscher, G. 1890. Die Hydroiden des K. K. Naturhistorischen Hofmuseums. Ann. K. K. Naturhist. Hofmus. 5: 195-286. [ Links ]
Marques, A.C. 1992. Sistemática dos Eudendriidae L.Agassiz, 1862 (Cnidaria, Hydrozoa) do Litoral Paulista. M. Sc. Thesis, Universidade de São Paulo. São Paulo. Brasil. 125 p. [ Links ]
McCrady, J. 1859. Gymnopthalamata of Charleston Harbor. Proc. Elliott Soc. Nat. Hist. 1: 103-221. [ Links ]
Mendel, M.D. & P.J. Lopez-Gonzalez. 1996. Updated catalogue of hydrozoans of the Iberian Peninsula and Balearic Islands, with remarks on zoogeography and affinities. In S. Piraino, F. Boero, J. Bouillon, P.F.S. Conelius & J.M. Gili (eds.). Advances in Hydrozoan Biology. Sci. Mar. 60: 183-209. [ Links ]
Meneghini, G. 1845. Osservazioni sullordine delle sertulariee della classe depolipi. Mem. Imp. Reale Inst. Veneto Sci., Lettere ed Arti 2: 183-199. [ Links ]
Migotto, A.E. 1993. Hidróides (Hydrozoa, Cnidaria) marinhos bentônicos da região costeira do canal de São Sebastião, SP. Ph. D. Dissertation, Universidade de São Paulo. Brasil. 258 p. [ Links ]
Millard, N.H.A. 1962. The Hydrozoan of south and west coasts of South Africa. Part I. The Plumulariidae. Ann South Afri. Mus. 46: 261-319. [ Links ]
Millard, N.H.A. 1966. The Hydrozoa of south and west coasts of South Africa. Part III. The Gymnoblastea and small families of Calyptoblastea. Ann. South Afr. Mus. 48: 427-487. [ Links ]
Millard, N.H.A. 1967. Hydroids from the south-west Indian Ocean. Ann. South Afr. Mus. 50: 168-194. [ Links ]
Millard, N.H.A. 1975. Monograph on the Hydroida of southern Africa. Ann. South Afr. Mus. 68: 1-513. [ Links ]
Millard, N.H.A. & J. Bouillon. 1973. Hydroids from the Seychelles (Coelenterata). Ann. Mus. Roy. Afr. Cent., sér. In-8º, Sci. Zool. 206: 1-106. [ Links ]
Morris, B.F. & D.D. Mogelberg. 1973. Identification manual to the pelagic Sargassum fauna. Bermuda Biol. Sta. Res. 11: 1-63. [ Links ]
Nutting, C.C. 1900. American hydroids. Part I. The Plumularidae. Smithsonian Institution, U. S. Nat. Mus., Spec. Bull. 4: 1-285. [ Links ]
Nutting, C.C. 1904. American hydroids. Part II. The Sertulariidae. Smithsonian Institution, U. S. Nat. Mus, Spec. Bull. 4: 1-325. [ Links ]
Nutting, C.C. 1905. Hydroids of the Hawaiian Islands collected by the steamer Albatross in 1902. Bull. U. S. Fish Comm. 1903: 931-959. [ Links ]
Nutting, C.C. 1915. American Hydroids. Section III. The Campanularidae and Bonneviellidae. Smithsonian Institution, U. S. Nat. Mus. Spec. Bull. 4: 1-126. [ Links ]
Orbigny, A. 1846. Voyage dans lAmerique Méridionale (le Brésil, la République orientale de luruguay, la République Argentine, la Patagonie, la République du Chili, la Republique de Bolivia, la République du Pérou), exécuté pendant les années 1826, 1827, 1828, 1829, 183, 1831, 1832 et 1833. Tome 5. 4e. Partie: Zoophytes. Levrault, Paris. 28 p. [ Links ]
Park, J.H. & B.J. Rho. 1986. A systematic study on marine hydroids of Korea. 9. The family Sertulariidae. Korean J. Syst. Zool. (Special Issue) 1: 1-52. [ Links ]
Pennycuik, P.R. 1959. Faunistic records from Queensland. Part V. Marine and brackish water hydroids. Papers Dep. Zool. Univ. Queensland 1: 141-210. [ Links ]
Petersen, K.W. 1979. Development of coloniality in Hydrozoa. p. 105-139. In G. Larwood & B. Rosen (eds.). Biology and Systematic of colonial organisms. The Systematic Association, Academic, New York. [ Links ]
Petersen, K.W. 1990. Evolution and taxonomy in capitate hydroids and medusae. Zool. J. Linn. Soc. 100: 101- 231 [ Links ]
Picard, J. 1951a. Note sur le hydraire littoraux de Banyuls-sur- Mer. Vie et Milieu. Banyuls-sur-mer 2: 338-349. [ Links ]
Picard, J. 1951b. Hydraires littoraux du Sénégal récoltés par H. Sourie aux environs de Dakar. Bull. Inst. Fondam. Afr. Noire 13: 109-115. [ Links ]
Pictet, C. 1893. Étude sur les hydraires de la Baie dAmboine. Rev. Suis. Zool. 1: 1-64. [ Links ]
Pictet, C. & M. Bedot. 1900. Hydraires provenant des campagnes de lHirondelle (1886-1888). Résultats des Campagnes Scientifiques Accomplies sur son Yacht par Albert I, Prince Souverain de Monaco 18: 1-59. [ Links ]
Ramil, F. & W. Vervoort. 1992. Report on the Hydroida collected by the "BALGIM" expedition in and around the Strait of Gibraltar. Zool. Verhandel. 277: 3-262. [ Links ]
Rees, W.J. & S. Thursfield. 1965. The hydroid collections of James Ritchie. Proc. Roy. Soc. Edinburg Biol. 69: 34-220. [ Links ]
Rees, W.J. & W. Vervoort. 1987. Hydroids from the John Murray Expedition to the Indian Ocean, with revisory notes on Hydrodendron, Abientinella, Cryptolaria and Zygophylax (Cnidaria:Hydrozoa). Zool. Verhandel. 237: 1-209. [ Links ]
Rees, W.J. & E. White. 1966. New records and fauna list of hydroids from the Azores. Ann. Mag. Nat. Hist. 9: 271-284. [ Links ]
Rho, B.J. & J.H. Park. 1986. A systematic study on the marine hydroids in Korea. 10. The family Plumulariidae. J. Korean Res. Inst. Better Living, Ewha Womans Univ. 37: 87-112. [ Links ]
Riedl, R. 1959. Die Hydroiden des Golfes von Naepel und ihr Anteil an der Fauna unterseeischer Höhlen. Pub. Sta. Zool. Napoli 30(Suppl.): 591-755. [ Links ]
Ritchie, J. 1908. On collections of the Cape Verde Islands marine fauna, made by Cyril Crossland, M.A. (Cantab.), B.Sc. (London), F.S.Z., of St. Andrews University, July to September, 1904. The hydroids. Proc. Zool. Soc. London 1907: 488-514. [ Links ]
Ritchie, J. 1909. Supplementary report on the hydroids of the Scottish National Antarctic Expedition. Trans. Nat. Roy. Soc. Edinburgh 47: 65-101. [ Links ]
Ritchie, J. 1910. Themarine fauna of the Mergui Archipelago, Lower Burma, collected by Jas. J. Simpson, M.A., B.Sc., and R.N. Rudmose-Brown, D. Sc., University of Aberdeen, February to May 1907. The hydroids. Proc. Zool. Soc. London 1910: 799-825. [ Links ]
Ritchie, J. 1911. Hydrozoa (hydroid zoophytes and Stylasterina) of the "Thetis" Expedition. Australian Mus. 4: 805-869. [ Links ]
Sars, M. 1850. Beretning om en i Lofoten og Finmarken. Nyt. Mag. Naturvidensk. 6: 121-211. [ Links ]
Sars, M. 1857. Bidrag til kundskaben om Middelhavets Littoral-Fauna, Reisebemaerninger fra Italien. Nyt Mag. Naturvidensk. 9: 110-164. [ Links ]
Schuchert, P. 1997. Review of the Family Halopteridae (Hydrozoa, Cnidaria). Zool. Verh. Leiden 309: 1-162. [ Links ]
Silveira, F.L. & Migotto, A.E. 1991. The variation of Halocordyle disticha (Cnidaria, Athecata) from the Brazilian Coast: an environmental indicator species? Hydrobiologia 216/217: 437-442. [ Links ]
Smaldon , G.D., D. Heppell & K.R.Watt. 1976. Type specimens of invertebrates (excluding insects) held at the Royal Scottish Museum, Edinburgh. Inf. Ser. R. Scot. Mus (Nat. Hist.) 4: i-iv, 1-118. [ Links ]
Smallwood, W.M. 1910. Notes on hydroids and nudi-branchs of Bermuda. Proc. Zool. Soc. London 1910: 137-145. [ Links ]
Souza, A.F. 1997. Sertulariídeos (Hydrozoa, Thecatae) das construções recifais do litoral norte do estado da Bahia. B. Sc. Thesis. Instituto de Biologia, Universidade Federal da Bahia, Brasil. 84 p. [ Links ]
Splettstösser, W. 1929. Beiträge zur Kenntnis der Sertulariden. Thyroscyphus Allm., Cnydoscyphus nov. gen., Parascyphus Ritchie. Zool. Jahrb. Abt. Syst. Ökol. Geogr. Tiere 58: 1-134. [ Links ]
Stechow, E. 1913a. Hydropolypen der japanischen Ostküste II. Teil: Campanulariidae, Halecidae, Lafoedidae, Campanulidae und Sertularidae, nebst Ergänzungen zu den Mathematich-Physikalischen Klasse der Königlichen Bayerischen Akademie der Wissenschften (Suppl.-Band 2) 3: 1-162. [ Links ]
Stechow, E. 1913b. Neue Genera thecater Hydroiden aus der Familie der Lafoeiden und neue Species von Thecaten aus Japan. Zool. Anz. 43: 137-144. [ Links ]
Stechow, E. 1914. Zur Kenntnis neuer oder seltener Hydroidpolypen, meist Campanulariden, aus Amerika und Norwegen. Zool. Anz. 45: 120-136. [ Links ]
Stechow, E. 1919. Zur Kenntnis der Hydroidenfauna des Mittelmeeres, Amerikas und anderer Gebiete, nebst Angaben über einige Kirchenpauersche Typen von Plumulariidae. Zool. Jahrb. Abt. Syst. Geogr. Biol. Thiere 42: 1-172. [ Links ]
Stechow, E. 1920. Neue Ergebnisse auf dem Gebiete der Hydroidenenforschung. Sitzungsb. Ges. Morphol. Physiol. Müchen 31: 9-45. [ Links ]
Stechow, E. 1921. Neue Genera und Species von Hydrozoen und anderen Evertebraten. Arch. Naturges. Abt. A. 3. Heft 87: 248-265. [ Links ]
Stechow, E. 1922. Zur Systematik der Hydrozoen, Stromatoporen, Siphonophoren, Anthozoen und Ctenophoren. Arch. Naturges. 88: 141-155. [ Links ]
Stechow, E. 1923a. Zur Kenntnis der Hydroidenfauna des Mittelmeeres, Amerikas und anderer Gebiete II. Teil. Zool. Jahrb. Abt. Syst Ökol. Geogr. Thiere 47: 29-270. [ Links ]
Stechow, E. 1923b. Neue Hydroiden der Deutschen Tiefsee-Expedition, nebst Bemerkungen über einige andre Formen. Zool. Anz. 56: 1-20. [ Links ]
Stechow, E. 1925. Hydroiden von West und Südwestaustralien nach den Sammlungen von Prof. Dr. Michaelsen und Prof. Dr. Hartmeyer. Zool. Jahrb. Abt. Syst. Geogr. Biol. Thiere 50: 191-270. [ Links ]
Stranks, J.N. 1993. Catalogue of recent Cnidaria type specimens in the Museum of Victoria. Occ. Papers Mus. Victoria 6: 1-28. [ Links ]
Summers, R.G. 1970. The fine structure of the spermatozoon of Pennaria tiarella (Coelenterata). J. Morph. 131: 117-130. [ Links ]
Summers, R.G. 1972. A new model for the structure of the centriolar satellite complex in spermatozoa. J. Morph. 137: 229-242. [ Links ]
Summers, R.G. & J.F. Haynes. 1969. The ontogeny of intersticial cells in Pennaria tiarella. J. Morph. 129: 81-88. [ Links ]
Svoboda, A. 1979. Beitrag zur Ökologie, Biometrie und Systematik der mediterranen Aglaophenia Arten (Hydroidea). Zool. Verhandel. 167: 1-114. [ Links ]
Svoboda, A. & P.F.S. Cornelius. 1991. The European and Mediterranean species of Agalophenia (Cnidaria: Hydrozoa). Zool. Verhandel. 274: 1-72. [ Links ]
Thornely, L.R. 1900. The hydroid zoophytes collected by Dr. Willey in the southern seas. p. 451-457. In A. Willey (ed.). Zoological results based on material from New Britain, New Guinea, Loyalty Islands and elsewhere, collected during the years 1895, 1896 and 1897. Part IV. Cambridge University, Cambridge. [ Links ]
Tizard, T.H., H.N. Moseley, J.Y. Buchanan & J. Murray. 1885. Narrative of the cruise of H. M. S. Challenger with a general account of the Scientific Resultats of the Voyage of H. M. S. Challenger during the Years 1873-76. Narrative 1: 1-509. [ Links ]
Torrey, H.B. 1902. The Hydroida of the Pacific coast of North America, with especial reference to the species in the collection of the University of California. Univ. Calif. Publ. Zool. 1: 1-104. [ Links ]
Totton, A.K. 1926. Note on rare Atlantic hydroid. Ann. Mag. Nat. Hist. 27: 210-212. [ Links ]
Van Beneden, P.J. 1867. Recherches sur la faune littorale de Belgique (polypes). Mém. Acad. Roy. Sci. Let. Beaux-Arts Belg. 36: 1-207. [ Links ]
Van Breemen, P.J. 1905. Plankton van Noord – en Zuiderzee. Tijdschr. Ned. Dierk. Vereenig.(2). 9: 145-324. [ Links ]
Van Germerden-Hoogeven, G.C.H. 1965. Hydroids of the Caribbean: Sertulariidae, Plumulariidae and Aglaopheniidae. Stud. Fauna Curaçao 22: 1-87. [ Links ]
Vannucci, M. 1951. Hydrozoa e Scyphozoa existentes no Instituto Paulista de Oceanografia. Bol. Inst. Paulista Oceanogr. 2: 69-100. [ Links ]
Vannucci-Mendes, M. 1946. Hydroida Thecaphora do Brasil. Arq. Zool. São Paulo 4: 535-597. [ Links ] Verrill, A.E. 1874. Report upon the invertebrate animals of Vineyard Sound and the adjacent waters, with an account of the physical features of the region. Rep. Comm. Fish. 1871-1872: 295-747. [ Links ]
Verrill, A.E. 1900. Additions to the Anthozoa and Hydrozoa of Bermuda. Trans. Connect. Acad. Arts Sci. 10: 551-572. [ Links ]
Versluys, J. 1899. Hydraires calyptoblastiques recueillis dans le Mer des Antilles, pendant lune des croisières accomplies par le comte R. de Dalmas sur son yacht CHAZALIE. Mém. Soc. Zool. France 12: 29-58. [ Links ]
Vervoort, W. 1941. Biological results of the Snellius Expedition. XI. The Hydroida of the Snellius expedition (Milleporidae and Stylasteridae excluded). Temminckia 6: 186-240. [ Links ]
Vervoort, W. 1959. The Hydroida of the tropical west coast of Africa. Atlantide Rep. 5: 211-325. [ Links ]
Vervoort, W. 1966. Bathyal and abyssal hydroids. Galathea Rep. 8: 97-174. [ Links ]
Vervoort, W. 1968. Report on a collection of Hydroida from the Caribbean region, including an annotated checklist of Caribbean hydroids. Zool. Verhandel. 92: 1-124. [ Links ]
Vervoort, W. 1972. Hydroids from the Theta, Vema and Yelcho cruises of the Lamont-Doherty Geological Observatory. Zool. Verhandel. 120: 1-247. [ Links ]
Vervoort, W. 1985. Deep-water hydroids: 267-297. In L. Laubier & C. Moniot (eds.). Peuplements profonds du golfe de Gascogne. Campagnes Biogas: 267-297. Institut Français de Recherche pour LExploration de la Mer (INFREMER), Brest. [ Links ]
von Lendenfeld, R. 1885. The Australian Hydromedusae. II. Proc. Linn. Soc. New South Wales 9: 345-363. [ Links ]
Wallace, W.S. 1909. A collection of hydroids made at the Tortugas, during May, June and July, 1908. Carnegie Inst. Wash., Year Book 7: 136-138. [ Links ]
Warren, E. 1906. On Halocordyle cooperi sp. Nov. a hydroid from the Natal Coast. Ann. Natal Govern. Mus. 1: 73-81. [ Links ]
Warren, E. 1908. On a collection of hydroids, mostly from the Natal coast. Ann. Natal Govern. Mus. 1: 269-355. [ Links ]
Weill, R. 1934. Contribuition à létude des cnidaires et de leurs nematocystes II. Valeur taxonomique du cnidome. Travaux de la station Zoologique de Wimereux 11: 351-701. [ Links ]
Weill, R. 1937. Existence dun monocnidome dans le médusoïde dun polype (Pennaria? Tiarella McGr) à tétracnidome. Compt. Rend. Hebdomad. Séanc. Acad. Sci. 204: 1479-1752. [ Links ]
Weismann, A. 1883. Die Entstehung der Sexualzellen bei den Hydromedusen. Zugleich ein Beitrag zur Kenntnis des Baues und der Lebenserscheinungen dieser Gruppe. Gustav Fisher, Jena. 295 p. [ Links ]
Werner, B. 1984. Stamm Cnidaria, Nessetiere. p. 11-305. In G. Hartwich, F. Killian, K. Odening. & B. Werner (eds). Lehrbuch der Speziellen Zoologie, Band I: Wirbellose Tiere 2. teil: Cnidaria, Ctenophora, Mesozoa, Plathelminthes, Nemertini, Entoprocta, Nemathelminthes, Priapulida. Gustav Fischer, Stuttgart. [ Links ]
Yamada, M. 1959. Hydroid fauna of Japanese sea and its adjacent waters. Publ. Akkeshi Mar. Biol. Sta. 9: 1- 101. [ Links ]