Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.49 n.3-4 San José Dec. 2001
Swimming ability in three Costa Rican dry forest rodents
William M. Cook 1 , Robert M. Timm 1 and Dena E. Hyman 2
1 Department of Ecology and Evolutionary Biology & Natural History Museum, Dyche Hall, University of Kansas, Lawrence, KS 66045 USA. Fax: (785) 864-5335. E-mail: wmcook@ku.edu.
2 Department of Biology, University of Miami, Coral Gables, FL 33124 USA.
Received 18-VII-2000. Corrected 20-III-2001. Accepted 30-III-2001.
Abstract
We investigated the swimming abilities of three Costa Rican dry forest rodents (Coues rice rat, Oryzomys couesi, hispid cotton rat, Sigmodon hispidus, and spiny pocket mouse, Liomys salvini) associated with a large marsh, Laguna Palo Verde, using 90 s swim trials in a plastic container. Swimming ability was evaluated by observing the use of limbs and tail in the water, inclination to the surface, and diving and floating behavior. Rice rats could float, swim and dive, suggesting that they can exploit surface and underwater resources. Cotton rats swam at the waters surface, but were less skilled swimmers than rice rats. Spiny pocket mice tired quickly and had difficulty staying at the waters surface. Results suggest that differential swimming ability is related to the distribution of the three sympatric species within the marsh and adjacent forest habitats.Key words: Dry forest, Liomys salvini, Oryzomys couesi, Sigmodon hispidus, swimming ability.
Most rodents can swim when necessary, and may even dive and swim skillfully (Dagg and Windsor 1972). Though dispersal over water is common (Carter and Merritt 1981, Forys and Dueser 1994, Giannoni et al. 1994), many species swim for other reasons. Most comparative analyses of swimming rodents focus on species from unrelated taxa (Schmidly and Packard 1967, Stock 1972, Hickman and Machiné 1986) or compare species from different habitats (Harris and Petersen 1979). As no comparative studies of sympatric species in the same microhabitat had been undertaken previously, we compared the wimming behavior of three sympatric marshassociated rodents. Three rodent species are found in Laguna Palo Verde and environs, Parque Nacional Palo Verde, Guanacaste Province, Costa Rica Coues rice rats (Oryzomys couesi), hispid cotton rats (Sigmodon hispidus), and spiny pocket mice (Liomys salvini). The Laguna is a large marsh with a seasonally variable water level, which in recent years has been dominated by dense stands of emergent cattails (Typha dominguensis). Coues rice rats are found from Texas to Colombia and typically inhabit marsh edges. Cotton rats range from central U.S.A. to Venezuela, and are found in wet and dry areas. Both rice rats and cotton rats nest above ground in grasses and standing vegetation. Spiny pocket mice are found in drier areas from southern Mexico to Costa Rica, and construct elaborate underground burrows (Reid 1997).
Via prior observations, morphology, and habitat preference, we predicted swimming ability in each species. Released rice rats and cotton rats swim briefly over water before disappearing into their native emergent vegetation; we expected that each could swim for at least a moderate period of time. While neither population in the study area has flattened tails or webbed feet, some mammals without obvious adaptations can still be very good swimmers (Blair 1939, Dagg and Windsor 1972). In general, we expected rice rats and cotton rats to swim or float during short trials as if crossing short gaps between floating vegetation mats. We did not expect spiny pocket mice to be adept swimmers as they also lack obvious morphological adaptations, are primarily a xeric species, and have not been observed in the water at our site.
Materials and methods
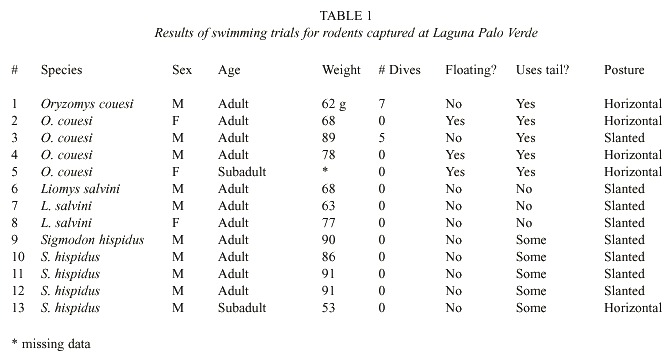
Animals in apparent distress were removed from the water prior to the end of the 90 s period. Swimming ability was evaluated by: 1) observed diving or floating behavior, 2) body inclination, 3) propulsive use of the tail, 4) leg positions while swimming, and 5) whether eyes were open underwater. We considered diving and swimming underwater, maintaining the bodys rear at the waters surface, use of the eyes underwater, and tail undulation as useful swimming skills. Species were categorized as possessing superior or inferior swimming ability based on the array of skills demonstrated by the tested individuals.
Results
Rice rats displayed strong swimming skills, calmly swimming around the arena. Two adult males repeatedly dove beneath the surface after 1 and 10 s respectively (see Table 1). Eyes were open at all times, and three individuals occasionally ceased active swimming and floated passively. Floating bouts began within a few seconds of immersion. All rice rats actively undulated their tails, and all but the heaviest individual (#3) kept a horizontal surface profile. All four legs kicked vertically in the water, though fore legs were occasionally inactive. Underwater propulsion was attributable to the tail and to the hind legs.Cotton rats swam adequately, but showed fewer skills than did rice rats. Except for animal #9, no individuals appeared in danger of sinking, but none dove or floated and the bodys posterior was typically below the surface (Table 1). All five animals used the tail for propulsion (but less vigorously than rice rats), and used all four legs (more actively than rice rats). Two cotton rats did not swim steadily throughout the trials–animal #9 was removed from the trial with 7 s remaining, and animal #10 repeatedly attempted to shake water off of its face. The most adept swimmer was the only female and subadult among the cotton rats tested: animal #13. It was able to keep its rear at the waters surface, although it did not dive or float.
As expected, spiny pocket mice gave little indication of swimming skill. All individuals kept their heads above water for some time, but this appeared to require considerable effort. No spiny pocket mouse dove or floated, and the animals were soon (1 min or less) oriented almost vertically in the water (Table 1).
All four legs were used to paddle, a technique that looked highly inefficient. No spiny pocket mice used the tail for propulsion, and they continually touched the edge of the container with their legs, seemingly trying to gain purchase. One animal (#8) had to be rescued 7 s before completion of the trial.
Discussion
Coues rice rat appears a highly adept swimmer, whose diving skills and propulsive tail use indicate that it may use underwater habitats for escape routes and foraging. While little is known about O. couesi, these observations are consistent with that which is known about its congener, O. palustris. In a morphometric analysis, Stein (1988) found O. palustris to be similar to the highly aquatic muskrats (Ondatra), citing elongation of the hind feet and tail, and water-repellant pelage in both taxa. While our population of rice rats lacks the laterally compressed tails present in muskrats, elongated rice rat tails are consistent with our observation of significant tail undulations. In Florida, Esher et al. (1978) observed O. palustris to swim underwater for up to 10 m, using an "exaggerated oscillation" of its tail, and individuals frequently floated at the waters surface without expending effort.Hispid cotton rats, while clearly not as skilled swimmers as rice rats, appear competent to disperse at the waters surface. Observations of released individuals indicate that cotton rats can pass readily between emergent vegetation patches. While our individuals used the tail for propulsion, they did not dive in trials or when released into the marsh; we cannot suggest that they use the underwater habitat. Our results are strongly consistent with prior evidence on swimming ability in Sigmodon and Oryzomys: Esher et al. (1978) observed cotton rats to swim across barriers when coerced, but they did not dive or float and were less likely to initiate water crossings than O. palustris. Similarly, Stein (1988) placed Oryzomys and Sigmodon on opposite ends of her "shape" principal component axis in a morphological study of semiaquatic murids.
Given above-water structure associated with cattails and aquatic Palo Verde trees (Parkinsonia aculeata), Laguna rice rats and cotton rats may have nest substrate, forage, and cover available on the interior of the marsh. As both species were captured up to 50 m away from shore (the maximum distance attempted), the marsh interior may support self-sustaining populations. Because both species have been captured in all marsh microhabitats and appear to be habitat generalists, we suggest that rice rats use of the underwater environment may help to differentiate the two species. This is consistent with Esher et al.s (1978) suggestion that advanced swimming skills in O. palustris contribute significantly to niche separation between it and cotton rats in Florida.
The third species found at Palo Verde, the spiny pocket mouse, is clearly a terrestrial animal. We did not expect spiny pocket mice to be adept swimmers, as they lack obvious morphological adaptations, and are well-known bur-rowers. This species was only captured on dry land at the edge of the marsh, and all individuals in trials could be considered poor swimmersat best. Spiny pocket mice do not use their tails while swimming, and their motions appear to be desperate and inefficient. This is consistent with Harris and Petersen (1979), who described L. irroratus performance as "lacking," and noted that all heteromyids they tested had to be rescued to prevent drowning. Most spiny pocket mice are not associated with aquatic habitats, and their swimming ability appears merely sufficient to survive accidental immersion.
The three rodents species associated with the Laguna Palo Verde fall on a gradient ofswimming ability. Coues rice rats can swim well at and below the waters surface and may be able to base their activities away from dry land. Hispid cotton rats are at home on land and on floating vegetation, and can readily cross gaps between clumps of emergent vegetation, but likely do not use the underwater medium. Spiny pocket mice are occasionally found at marsh edges, but not typically in aquatic habitats and cannot swim effectively. Further study in this system could involve formal measurements of aquatic behavior in rice rats and cotton rats, and study of their foraging and nesting requirements. This information could reveal more about niche differentiation between these sympatric species.Finally, much of the emergent vegetation used by rice rats and cotton rats did not exist in Laguna Palo Verde as recently as 1985, so local rodents have much more available habitat than in the past. As marshes such as Palo Verde fill with silt or become overgrown by cattails (possibly due to human activity), we can expect the abundance and distribution of native rodents to change along with the structure of their habitats.
Acknowledgments
We thank the Ministerio del Ambiente y Energía and Sistema Nacional de Áreas de Conservación for allowing us to work at Parque Nacional Palo Verde, and especially Javier Guevara S. for facilitating research permits. Eugenio González, Deedra McClearn, and the Organization for Tropical Studies provided logistical support for this project. Particular thanks go to Daniel Ardia, Jenny Drnevich, Andrea Huberty, and Jeremy Zujko-Miller for field assistance.
Resumen
Nosotros investigamos las habilidades de nado de tres ratones del bosque seco de Costa Rica (la rata arrocera de Coue, Oryzomys couesi, la rata algodonera híspida, Sigmodon hispidus, y el ratón espinoso, Liomys salvini) asociados a un gran pantano, Laguna Palo Verde, usando pruebas de nado de 90 s en un contenedor de plástico. La habilidad de nado fue evaluada observando el uso de las extremidades y cola en el agua, inclinación hacia la superficie y comportamiento de flotar y buceo. Las ratas arroceras pudieron flotar, nadar y bucear, sugiriendo que ellas pueden explotar los recursos en la superficie y bajo el agua. Las ratas algodoneras nadaron en la superficie del agua, pero fueron menos hábiles nadadoras que las rata arroceras. Los ratones espinosos se cansaron rápidamente y tuvieron dificultad para mantenerse en la superficie del agua. Los resultados sugieren que la habilidad de nado diferencial está relacionada con la distribución de las tres especies simpátricas dentro del pantano y los hábitats del bosque adyacentes.
References
Blair, W.F. 1939. A swimming and diving meadow vole. J. Mammal. 20: 375. [ Links ]
Carter, J.L. & J.F. Merritt. 1981. Evaluation of swimming ability as a means of island invasion by small mammals in Coastal Virginia. Ann. Carnegie Mus. 50: 31–46. [ Links ]
Dagg, A.I. & D.E. Windsor. 1972. Swimming in northern terrestrial mammals. Can. J. Zool. 50: 117–130. [ Links ]
Esher, R.J., J.L. Wolfe & J.N. Layne. 1978. Swimming behavior of rice rats (Oryzomys palustris) and cotton rats (Sigmodon hispidus). J. Mammal. 59: 551–558. [ Links ]
Forys, E.A. & R.D. Dueser. 1994. Inter-island movements of rice rats (Oryzomys palustris). Amer. Midl. Natur. 130: 408-412. [ Links ]Giannoni, S.M., C.E. Borghi & J.P. Martínez-Rica. 1994. Swimming ability of the Mediterranean pine vole Microtus (Terricola) duodecimcostatus. Acta Ther. 39: 257–265. [ Links ]
Harris, C.E. & M.K. Petersen. 1979. Comparative swimming performances in selected cricetid and heteromyid rodents. Occas. Pap. Zool. Michael K. Petersen 3: 1–16. [ Links ]Hickman, G.C. & C. Machiné. 1986. Swimming behaviour in six species of African rodents (Cricetidae, Muridae). Acta Ther. 31: 449–466. [ Links ]
Reid, F.A. 1997. A field guide to the mammals of Central America and southeast Mexico. Oxford University, New York. 334 p. [ Links ]
Schmidly, D.J. & R.L. Packard. 1967. Swimming ability in pocket mice. Southwest. Natur. 12: 480–482. [ Links ]
Stein, B.R. 1988. Morphology and allometry in several genera of semiaquatic rodents (Ondatra, Nectomys, and Oryzomys). J. Mammal. 69: 500–511. [ Links ]
Stock, A.D. 1972. Swimming ability in kangaroo rats. Southwest. Natur. 17: 98–99. [ Links ]














