Revista de Biología Tropical
versão On-line ISSN 0034-7744versão impressa ISSN 0034-7744
Rev. biol. trop vol.49 no.3-4 San José Dez. 2001
Pseudostigmatidae) with notes on their phenology and life zone preferences
Ingemar Hedström 1,3 and Göran Sahlén 2, 4
1 Department of Applied Science, Mid Sweden University, SE-871 88 Härnösand, Sweden
2 Systematic Zoology, Evolutionary Biology Centre, Uppsala University, Norbyvägen 18d, SE-752 36 Uppsala, Sweden
3 Present address: Escuela de Biología, Universidad de Costa Rica, 2060 San José, Costa Rica; and Department of Ecumenical Research, Apdo 390, 2070 Sabanilla, Costa Rica
4 Present address: School of Business and Engineering, Halmstad University, P. O. Box 823, SE-301 18 Halmstad, Sweden
Received 18-IX-2000. Corrected 06-II-2001. Accepted 08-11-2001.
Abstract
We present a key to the Costa Rican species of Pseudostigmatidae, comprising three genera with the following species: Megaloprepus caerulatus, Mecistogaster linearis, M. modesta, M. ornata and Pseudostigma aberrans. Pseudostigma accedens, which may occur in the region, is also included. For each species we give a brief account of morphology, phenology and life zone preferences, including distributional maps based on more than 270 records. These are not all of the known specimens from the area, but a high enough number to give a relatively good picture of the distribution and status of the species. We found M. caerulatus to be active during the first half of the year in seasonal, tropical semidry lowland forest and tropical moist forest at mid-elevation, but like M. linearis, M. caerulatus was active all year round in non-seasonal, tropical wet lowland forest and tropical moist forest at mid-elevation. Mecistogaster modesta also flew year round in non-seasonal, tropical wet lowland forest and tropical moist evergreen forest at mid-elevation, and likewise in seasonal and non-seasonal, tropical premontane moist forest. Only a few findings, however, have been made of M. modesta in seasonal, tropical semi-dry decidu-ous forest and seasonal, tropical moist evergreen forest. Mecistogaster ornata was missing entirely from non-seasonal, tropical wet lowland forest and non-seasonal, tropical moist forest at mid- elevation, while this species was active year round in seasonal, tropical dry lowland forest and tropical semi-dry forest, as well as in seasonal, tropical moist evergreen forest and tropical premontane moist forest, both at mid-elevation. Pseudostigma aberrans has so far been found too few times in Costa Rica for any indication of flight time preference.
Key words: Odonata, damselflies, Pseudostigmatidae, key, taxonomy, life zone preferences, phenology, status.
Pseudostigmatidae is a small Neotropical family of long-lived giant damselflies, which are characterised by their relatively slow flight and a very long and slender abdomen. Their large size and unusual feeding habits caught the attention of the early twentieth century naturalists (Calvert 1901-1908, 1911, 1923, Calvert and Calvert 1917). Unlike most adult odonates which catch flying insect prey, the giant damselflies orient to non-moving prey, e.g. small web-building spiders (Fincke 1984, 1992a, b, Rüppell and Fincke 1989b). On several occasions the authors have observed Mecistogaster modesta, apparently feeding on what may be small invertebrates sitting on leaves in sunlit areas. Megaloprepus caerulatus was observed feeding on a small damselfly (Argia sp.) on Barro Colorado Island (BCI), Panama (O.M. Fincke pers. comm.).
Whereas most odonates congregate at breeding sites such as ponds or streams (c.f. Corbet 1999), pseudostigmatids wander widely throughout the forest searching for waterfilled tree holes or tank bromeliads where females lay their eggs (Calvert 1901-1908, 1911, 1923, Calvert and Calvert 1917, Fincke 1998). Water containers may form in rotting burls, the crotch of branches, or in convolutions of the trunk of a fallen tree (Fincke 1992b) as well as in large bromeliads. The long abdomens of the Psedostigmatidae have been thought to be adaptations for egg-laying in tree holes and tank bromeliads (Calvert 1911) or to provide mechanical advantages for throwing eggs into such holes as observed by Machado and Martinez (1982). Although such habits may have selected for long abdomens, this is probably not the only explanation, because males of several species (see below) have relatively longer abdomens than females. In one of the species, Mecistogaster linearis, sexual selection via male-male competition has probably contributed to the evolution of long abdomens (Fincke 1984).
There are three genera of the giant damselfly family, commonly referred to as "helicopter" damselflies in Costa Rica: Megaloprepus Rambur, 1842 (one species), Mecistogaster Rambur, 1842 (three species) and Pseudostigma Sélys, 1860 (one, possibly two species). These six species are the only representatives of the family in Central America as a whole (Tsuda 1991).
The keys provided by Calvert (1901-1908) tend to be difficult to obtain and with the exception of the works of May (1979) and the compilation by Förster (1999), no modern keys are available to identify members of this family in Costa Rica or any other Central American country. Because a lot of ecological work has been done on some of the species, we feel that a new, illustrated key for both field and laboratory use would be of value and hopefully also generate further research on these damselflies. Our aim is also to use the available records as a base for presenting the phenology and distribution of the species in Costa Rica, focusing on different life zones.
Materials and methods
This paper is based on the authors observations and collections in Costa Rica in 1995 and between 1997-2000. Additional information was obtained by examining public and private collections in Costa Rica. Records from collections or field notes have also been supplied from many colleagues around the world; all in all some 270 records. This paper by no means gives a complete account of all records from Costa Rica, but we feel we have enough records available to prepare a first generation of distribution maps.
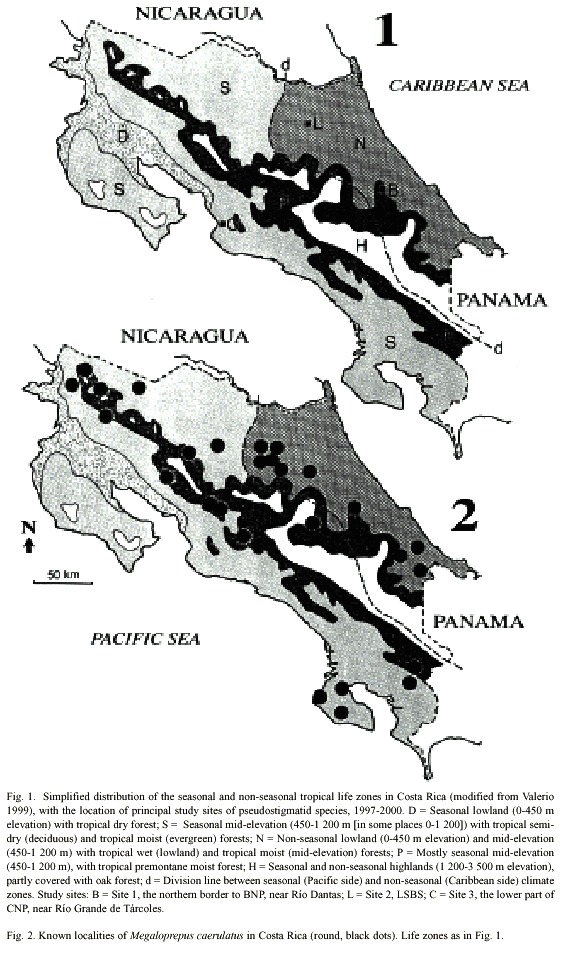
In this paper we adapted the life zones of Valerio (1999), to present preferences of the species (Figs. 2- 4). The zones used are: D) Seasonal lowland (0-450 m elevation) with tropical dry forest; S) Seasonal low to mid-elevation (450-1 200 m [in some areas 0-1 200]) with tropical semi-dry (deciduous) and tropical moist (evergreen) forests; N) Non-seasonal lowland (0-450 m elevation) and mid-elevation (450-1 200 m) with tropical wet (lowland) and tropical moist (mid-elevation) forests; P) Mostly seasonal mid-elevation (450-1 200 m), with tropical premontane moist forest; and H) Seasonal and non-seasonal highlands (1 200-3 500 m elevation), partly covered with oak forest; Fig. 1, cf. Holdridge (1967).
We concentrated our own field-work on three principal study sites. Two of these are in zone N, the third in zone S. The first site (Fig. 1: B) is located between 200-450 m elevation on the northern border to Barbilla National Park (BNP), near Río Dantas (10º00N; 83º26W), where we have data from 1994, although mostly from January and February each year. In this study area near Río Dantas, between 1986-1995, the maximum and minimum temperatures oscillated between 18-35 ºC (Edman and Hedström 1999, cf. also DeVries 1987, Hedström 1991 and Valerio 1999 for further information on the climate of the Caribbean slope). The second site (Fig. 1: L), La Selva Biological Station (LSBS; 10º26N; 83º59W), is also within a warm and wet lowland landscape (35 m elevation at the Station), with an average of 4 000 mm of rainfall that is spread evenly throughout the year (McDade et al. 1994). LSBS is relatively wellknown, with numerous pseudostigmatid records going back more than 30 years; the mean annual temperature oscillates between 21-31 ºC (LSBS, unpubl. data). The third site is in the lower part of Carara National Park (CNP; 09º46N; 84º36W; Fig. 1: C), where we have noted pseudostigmatids at different times of the year starting in 1997. The mean annual temperature at the site oscillates between 25-30 ºC (Carara Ranger Station, unpubl.), and the landscape is protected from the trade winds and their drying effect by a high mountain chain to the north. Climatology of the Pacific slope is described by Valerio (1999), but cf. also DeVries (1987) and Hedström (1991).
The life zone preferences of each species (except the two Pseudostigma spp.) were tested by a simple X2 -test (Zar 1999) where each life zone where the species had been found was given equal expected values.
The ink drawings in the key were produced using a stereo microscope fitted with a line drawing mirror. To depict wings we simply used a high-resolution flatbed scanner on the wing itself, resulting in a reproducible black and white image. Distribution maps were drawn in the old fashioned way using Letraset symbols before scanning. In the key and species descriptions, systematics follows Tsuda (1991). Wing vein terminology after Carle (1982).
Size measurements were generated from our own material plus museum material in Costa Rica and Sweden. Figs. from other papers were used in case we had access to fewer than five Costa Rican specimens. We measured between 20 and 30 specimens except in M. linearis (13), M. ornata (10), and the two Pseudostigmas (only 3 each). In the latter four species we included measurements from literature where such were available.
In the Records section, information has been reduced using abbreviations. Apart from those defined in the text, a list of further abbreviations is found after the Species Diagnoses section.
Results and discussion
Key to species
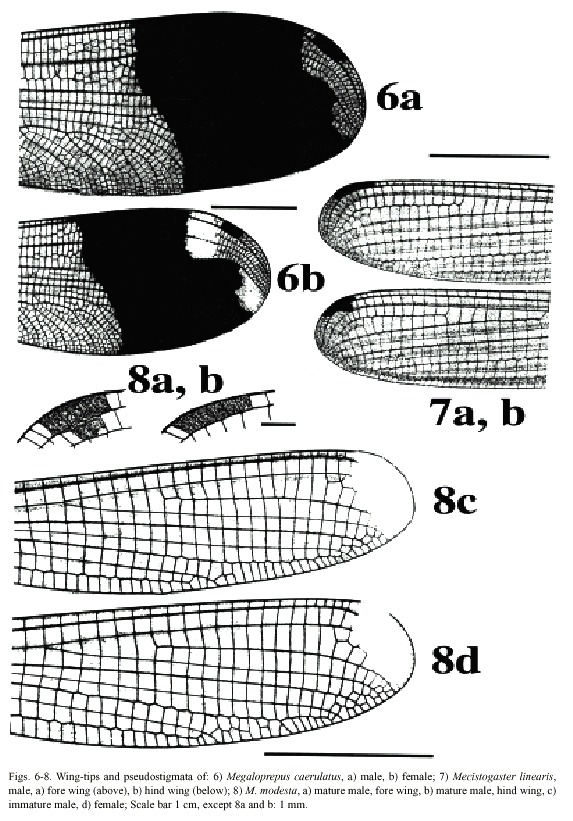
(1) Wings broad, with a dark, rectangular pseudostigma surrounded by an irregular, transparent area (Fig. 6a, b). A broad, dark blue band, shimmering in metallic, covers the entire width of the wing from around the branching of RP out to the clear area surrounding the pseudostigma. In males, the transparent area proximal to the dark band is milky white; in females this milky
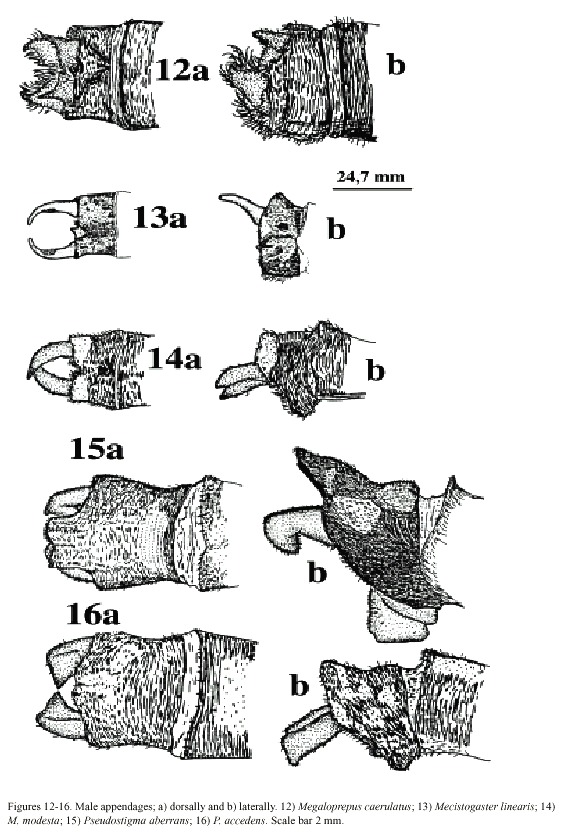
colour is present in the area at the wing tip; wing tips clear in males. Vein A 1 branched, resulting in numerous rows of cells between A1 and posterior margin of wing. Male superior appendages shorter than the inferior (Fig. 12a, b). . . . . . . . . . . . . . . . . . . . . . .Megaloprepus caerulatus
- Wings narrower, pseudostigma and wing-coloration different than above. Vein A1 not branched with only one or two rows of cells between A1 and posterior margin of wing (Figs. 7-11). Male superior appendages longer than the inferior (Figs. 13-16) . . . . . . . . . . . . . . . . .. . . . . . . . . . . . ..(2)
(2) Only one row of cells between A1 and posterior margin of wing (Figs. 7-9). Male abdominal segment 10 as high as wide. Male superior appendages forcipate, softly incurved (Figs. 13-14) . . . . . . . . . .Mecistogaster (3)
-Two rows of cells between A1 and posterior margin of wing, at least in middle section of wing (Figs. 10-11). Male abdominal segment 10 higher than wide, elongated dorsally. Male superior appendages strongly incurved
(Figs. 15-16) . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Pseudostigma 1 (5)
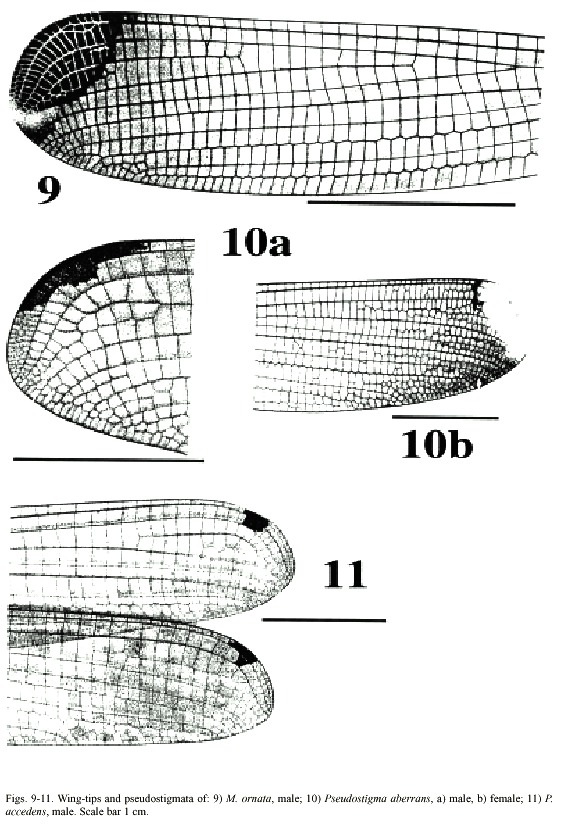
(3) Wing tips with yellow marking covering most of the pseudostigma (Fig. 9). In sexually mature males the yellow markings turn gradually dark brown, at least ventrally. Male superior appendages often lightly coloured except dark area dorsally close to the tip. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .M. ornata
-Wing tips transparent (most mature individuals) or milky white (most young individuals); pseudostigma covering two rows of cells in the fore wings and one row in the hind wings (Figs. 7-8) . . . . . . . . . . . . . . . .(4)
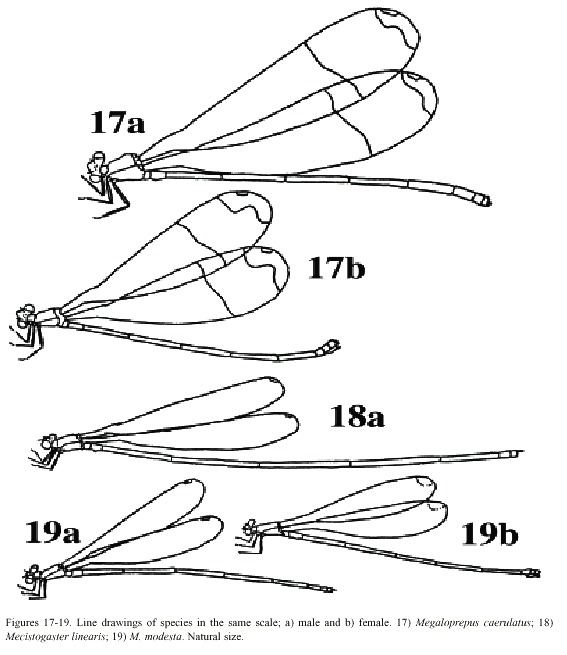
(4) Larger species; hind wing more than 50 mm in length. Young individuals of both sexes with milky wing tips and white pseudostigmata; these turn red, brown, or black in mature individuals (which have clear, sometimes smoky, wing tips; Fig. 7). Superior appendages in male (Fig. 13a, b) black. Abdomen long; more than 80 mm (Fig. 18) . . . . . . .M. linearis
- Smaller species; hind wing less than 47 mm in length. Young individuals of both sexes have milky wing tips and white pseudostigmata (Fig. 8c, d); these turn red brown in mature males only (Fig. 8a, b); males with clear wingtips. Superior appendages in male (Fig. 14a, b) normally with light base and black tips. Abdomen shorter; less than 75 mm (Fig. 19) . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .M. modesta
(5) Tips of male superior appendages bent downward; inferior appendages blunt but clearly distinguishable from the side (Fig. 16b). Female wing tips with yellow spot reaching to vein RP2 (Fig. 10b) . . . . . . . . . . . P. aberrans
- Points of male superior appendages not bent downward; inferior appendages very small, almost rudimentary (Fig. 17a, b). Female wing tips with yellow spot not reaching vein RP 2 . . . . . . . . . . . . . . . . . . . . . . . . . . .P. accedens
Species Diagnoses
All species have the same basic colour pattern with a dark ground colour and light markings forming a shoulder line and sidelines on the pterothorax. Important characters are wing markings and shape of male appendages. Distribution in Costa Rica is given in Figs. 1-4, where as the different life zones in the country are explained in Fig. 1.
Megaloprepus caerulatus (Drury, 1782)
Morphology: Total size large (Fig. 17). Body colour dark brown to black with yellowish markings. Wings wide with black or dark brown venation. Dark pseudostigma of 8-12 cells covering two rows (Fig. 6a, b), similar in fore- and hind wing, always surrounded by a transparent section. Proximal to this, a dark marking, reflecting light in blue, green and violet, stretches across the wing, ending near the branching of RP. In males a milky tinge is present on the inner section of the wing; in females this applies to the area round the pseudostigma. Male appendages dark, short and pointed (Fig. 12a, b), the inferior pair incurved; longer than the superior. Abdominal length in male (in mm): 72-104; female 66-84; hind wing in male 61-87; female 54-70.
Phenology and life zone preferences: Megaloprepus caerulatus occurs in primary mature wet and moist forests from southern Mexico-Belize to Bolivia, including Venezuela and Guyana (Calvert 1901-1908, Calvert and Calvert 1917, Tsuda 1991).
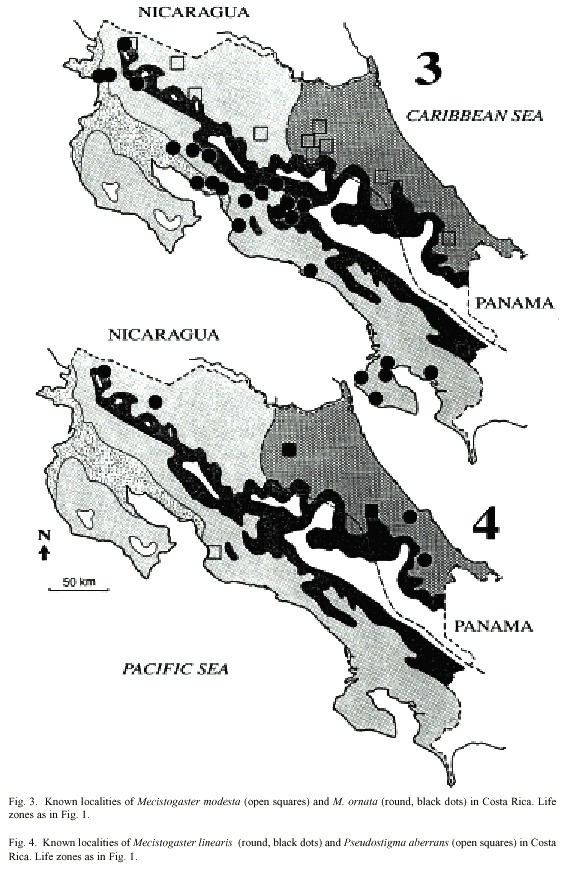
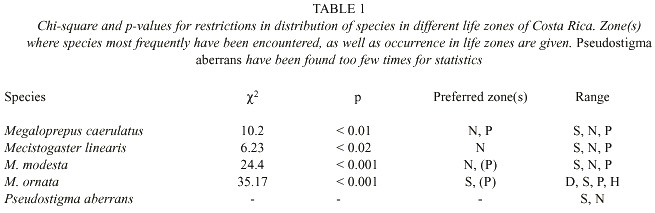
In Costa Rica, the species inhabits evergreen forest in zones S, N and P, up to 1500 m elevation (Table 1; Fig. 2). Most of the collection records and observations come from the Caribbean lowlands (N; Fig. 5). We observed the species in the primary forest of BNP and its surroundings, between 200 and 400 m elevation. Apart from the Osa Peninsula in the south, the species is notably absent from coastal areas and seems to follow the mountain range from south east to north west (Fig. 2).
Because M. caerulatus seems to avoid large, man-made clearings, it may be particularly vulnerable to habitat fragmentation (Fincke 1998). With the strong advancing deforestation, the number of populations in Costa Rica should continually diminish. The species is conspicuous and thus easily detected. The number of both new and old observations indicate that the species is still moderately common in certain primary rain forest areas.
Adult M. caerulatus have been found around the year in zones N and P, but only from January to July in zone S (Fig. 5). Thus it seems as if M. caerulatus is on wing mostly during the dry season in zone S, an interesting adaptation to a seasonal habitat. Megaloprepus caerulatus significantly prefers zones N and P to zone S (2 = 10.2, p < 0.01; Table 1).
Extensive ecological work has been done on this species on BCI by Fincke (e.g. 1984, 1992b, 1994, 1997, 1999) and coworkers, in particular on sexual selection and competitive exclusion.
Records: 1 m, 30 VI 1963, Rincón de Osa, Puntarenas P, ST; 1 m, 1 f, 4 VIII 1964, 4 km S Pandora, Limón P, TH; 1 m, 1 f, 26 VI 1965, Roxana, Limón P, NO; 1 m, 14 VII 1965, Turrialba, Cartago P, NA; 1 m, 1 f, 18 IX 1966, LSBS (60 m), 1 km S Puerto Viejo, Heredia P, MP; 1 m, 19 IX 1966, LSBS, Heredia P, MP; 1 m, 1 f, 20 IX 1966, LSBS, Heredia P, MP; 1 m, 21 IX 1966, LSBS, Heredia P, MP; 1 m, 22 IX 1966, LSBS, Heredia P, MP; 1 m, 1 f, 23 IX 1966, LSBS, Heredia P, MP; 1 f, 2 XI 1966, Bosque Florencia (600 m), stream, 5 km SE Turrialba, Cartago P, MP; 1 m, 18 III 1967, Rincón de Osa, Puntarenas P, DP; 1 m, 1 f, 9 IV 1967, LSBS, Heredia P, MP; 1 m, 12 IV 1967, LSBS, Heredia P, MP; 1 m, 19 IV 1967, 6.5 km S San Vito (1 200 m), Puntarenas P, BE; 1 m, 26 IV 1967, streams + forest, 6.5 km S San Vito (1 200 m), Puntarenas P, MP; 2 m, 14 V 1967, streams + waterfalls, 16 km N Vacablanca (850 m), Alajuela P, MP; 1 m, 1 f, 24 VI 1967, streams + waterfalls, 16 km N Vacablanca (850 m), Alajuela P, MP; 1 m, 1 VII 1967, LSBS, Heredia P, MP; 3 m, 2 VII 1967, LSBS, Heredia P, MP; 1 m, 12 VIII 1967, LSBS, Heredia P, MP; 1 f, 13 VIII 1967, LSBS, Heredia P, MP; 1 m, 14 VIII 1967, LSBS, Heredia P, MP; 1 f, 14 II 1968, Rincón de Osa vic, Puntarenas P, BE; 1 m, 1 f, 12 III 1969, Rincón de Osa, Playa Blanca Road, Puntarenas P, DP; 1 f, 9 III 1970, Rincón de Osa vic, Puntarenas P, DP; 1 f, 20 VII 1970, Monteverde (1 420 m), Puntarenas P, BU; 1 m, 21 VI 1972, forest trail, Monteverde, Puntarenas P, SL; 1 m, 2 VI 1978, Cataratas de San Ramón, Alajuela P, CC; Several individuals, 13 III 1980, Corcovado NP, Puntarenas P, DN; 1 m, 1 f, 30 I 1982, Chachagua, San Carlos, Alajuela P, MA; 1 m, 3 VI 1982, Chachagua, San Carlos, Alajuela P, MA; 1 m, 3 VII 1982, Chachagua, San Carlos, Alajuela P, OM; 1 m, 1 VI 1984, Reserva de San Ramón, Alajuela P, AN; Several individuals, 10-11 VI 1986, 9 km SW Pandora, Limón P, DN; 1 m, 29 III 1987, LSBS, Heredia P, BL; 1 m, 31 X 1987, Pizote, Dos Ríos, Alajuela P, GL; 1 m, 2 XII 1987, Hacienda Santa María (840 m), Rincón de la Vieja NP, Guanacaste P, GL; 1 m, 4 IX 1988, Chilamate, W of Puerto Viejo, Heredia P, EL; 1 m, 1 f, 28 XI 1988, LSBS, Heredia P, ES; 1 f, 8 XII 1988, El Rodeo Forest Preserve, Colón, San José P, ES; 1 f, 8 I 1989, Colón, San José P, CH; 1 f, 16 III 1989, Pitillo BS (450 m), Guanacaste NP, Guanacaste P, VA; 1 f, 30 XI 1989, San Vito, Coto Brus, Puntarenas P, R. MG; 1 m, 9 IX 1990, near Río Cerere, Hitoy Cerere BR, Limón P, ZU; 1 m, 19 IX 1990, Magsasay BS, Braulio Carrillo NP, Heredia P, ZU; 1 specimen, III 1991, Pitillo BS, Guanacaste P, JO; 1 m, 1 f, 23 VII 1991, Maritza BS (collected while copulating 2 m above the water of small creek), Santa Rosa NP, Guanacaste P, RA; 1 m, 17 III 1993, Upala, Río Aguas Verdes, Alajuela P, RA; Several individuals, 24-28 III 1995, 10 km SW Horquetas, Heredia P, DN; 1 m, 14 IV 1995, near Río Dantas, BNP, Limón P, HE; 1 m, 3 V 1995, Jardín Botánico Wilson, Coto Brus, Puntarenas P, RA; 1 m, 18 V 1995, Coto Brus, Puntarenas P, CH; 1 m, 12 VIII 1995, Las Alturas de Cotón, San Vito, Coto Brus, Puntarenas P, CN; 2 m, 21 VIII 1995, LSBS, Heredia P, CA; 1 f, 21-28 VIII 1996, near Río Dantas, BNP, Limón P, GP; 1 f, 1-9 IX 1996, Guanacaste NP, trail between Maritza BS and Cacao Field Station (600-900 m elevation) , Guanacaste P, VK; 1 f, 2-8 IX 1996, Guanacaste NP, Maritza BS, Casafran trail (600-1 000 m elevation), Guanacaste P, GP; 1 m, 28 I 1997, near Río Dantas, BNP, Limón P, HE; 9 m, 4 f, 26 I - 2 II 1998 (11.20 a.m, 1 pair in tandem), near Río Dantas, BNP, Limón P, HG; 9 m, 1 f, 10-22 I 1999, LSBS, Heredia P, sighting. SO; 3 m, I 1999, SBS, Heredia P, sighting. SO; 1 m, 1 emerging teneral, 24 I 1999, Corcovado NP (on the trail from San Pedrillo to La Llorona), Puntarenas P, sighting. SO; 15 m, 3 f, 16 II 2000, LSBS, Heredia P, sighting. SO; 1 f, 23 IV 2000, near Río Dantas, BNP, Limón P, HE; 2 m, 23 V 2000, near Río Dantas, BNP, Limón P, HE; 1 m, 3 VI 2000, near Río Dantas, BNP, Limón P, HG; 3 m, 4 VI 2000, near Río Dantas, BNP, Limón P, HG; 1 m, 5 VI 2000, near Río Dantas, BNP, Limón P, HG; 1 m, 8 VI 2000, near Río Dantas, BNP, Limón P, HG.
Mecistogaster linearis (Fabricius, 1776)
Morphology: Total size large, but narrow wings make the species less massive than M.
caerulatus (Fig. 19). Body colour very dark brown; light markings often a whitish brown. Wings with dark venation; clear with a small brown pseudostigma covering 8-12 cells in two rows in the fore wing; a single row in the hind wing (Fig. 7a, b). Male superior appendages long and forcipate, mostly dark with lighter areas (Fig. 13a, b); inferior appendages small and blunt. Abdominal length in male (in mm): 105-117; female 81-96; hind wing in male 52-59; female 52-60.
Phenology and life zone preferences: Mecistogaster linearis is primarily a South American species, distributed from Argentina and Brazil up to Venezuela, Peru and Ecuador (Tsuda 1991). The species inhabits primary and secondary forests in Costa Rica and Panama, and prefers to forage in semi-shaded areas (Fincke 1984, 1992a).
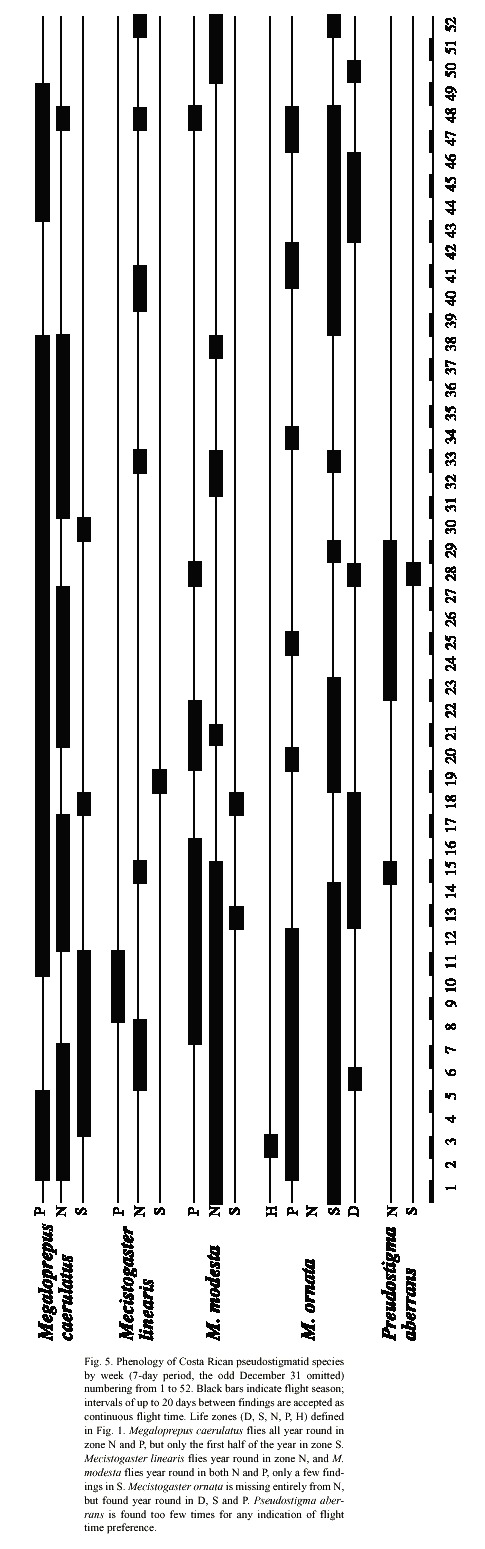
In Costa Rica, M. linearis has been found almost all year round, but only some 15 specimens are known to us, all found on a total of six localities (Fig. 4). All findings are situated in the lowlands north east of the mountains in zones N and S; rarely in P; the northernmost finding at Pitillo Biological Station, Guanacaste Province, relatively close to the Nicaraguan border (Fig. 4). The species is notably uncommon in Costa Rica preferring zone N to S and P (2 = 6.23, p < 0,02; Table 1; Fig. 5).
Records: 1 m, near Río Reventazón (50 m a.s.l), Limón P, UN; 1 m, 21 II 1976, 5 km W Portete, Limón, Limón P, DP; 1 m, 10 IV 1976, LSBS, 2.5 km S Puerto Viejo, Heredia P, MP; 1 m, 14 IV 1976, LSBS, Heredia P, MP; 1 f, 14 VIII 1976, LSBS, Heredia P, MP; 1 m, 1979, LSBS, Heredia P, ES; 1 f, 12 V 1988, La Garroba, Guatuso, Alajuela P, GS; 1 f, 1 m, 28 XI 1988, LSBS, Heredia P, ES; 1 m, 1-16 III 1989, Pitillo BS (450 m), Guanacaste NP, Guanacaste P, VA; 1 m, 6 X 1991, Hitoy Cerere BR, Limón P, CA; 1 m, 9 X 1991, Hitoy Cerere BR, Limón P, CA; 1 m, II 1999, near Río Dantas, BNP, Limón P, HE; 1 m, 28 XII 1999, near Río Dantas, BNP, Limón P, HE; several m, f, 7-18 II 2000, LSBS, Heredia P, sighting, SO.
Mecistogaster modesta Selys, 1860
(Figs. 3, 8a, b, c, d, 14a, b, 19)
Morphology: The smallest species of Pseudostigmatidae of the area (Fig. 19). Dark in colour, often pure black; light areas beige, sometimes yellow. Wings clear with a small pseudostigma covering two rows in the fore wing; a single row in the hind wing (Fig. 8a-d). Pseudostigma white in young males and females, turning red or brown in mature males. Amilky tinge present at the wing-tips of young individuals. Male superior appendages evenly forcipate with the tips bent just slightly down-ward, dark in colour. Inferior appendages short, slightly pointed, clearly visible when viewed laterally (Fig. 14a, b). Abdominal length in male (in mm): 63-70; female 58-71; hind wing in male 41-43; female 39-46.
Phenology and life zone preferences: Mecistogaster modesta inhabits wet and moist primary forests from Mexico-Belize to Colombia and Venezuela (Tsuda 1991, Paulson 1997). Most of the Costa Rican records of M. modesta are from November to May, with peak observations in January and March, the less rainy months of the year on the Caribbean slope. The species have been found all year round in zones N and P with only a few records in zone S during March and April (Fig. 5). The species exhibits a significant preference to zone N (to some extent also P) (2 = 24.4, p < 0.001; Table 1). Given that the species is rather small, yet quite common in collections, its status in Costa Rica is probably "locally common". The known distribution is shown in Fig. 3. Mecistogaster modesta was first recorded from Costa Rica by Calvert (1911), who suggested that the species oviposits in tank bromeliads or water-filled tree-holes. Fincke (1998) commonly found M. modesta larvae in bromeliads, but observed none in any of the tree-holes, husks or palm fronds sampled, corroborating Calverts (1911) conclusion that this species is a bromeliad specialist. Seven of 20 bromeliad leaf axils sampled in LSBS harboured a M. modesta larva, two of these were final instars, both over 17 mm in total length (Fincke 1998).
Records: 1 m, 24-28 III year not known, SW Horquetas (600 m), Heredia P, DN; 2 m, 24-28 III year not known, Guanacaste NP, Guanacaste P, DN; 1 m, 19 IX 1966, LSBS, 2.5 km S Puerto Viejo, Heredia P, MP; 1 f, 21 IX 1966, LSBS, Heredia P, MP; 1 f, 22 IX 1966, LSBS, Heredia P, MP; 1 m, 1 f, 9 IV 1967, LSBS, Heredia P, MP; 1 f, 10 IV 1967, LSBS, Heredia P, MP; 1 m, 1 f, 12 IV 1967, LSBS, Heredia P, MP; 1 m, 13 IV 1967, LSBS, Heredia P, MP; 1 m, 14 V 1967, streams + waterfalls 16 km N Vacablanca (850 m), Alajuela P, MP; 1 m, 12 VIII 1967, LSBS, Heredia P, MP; 1 m, 1 f, 13 VIII 1967, LSBS, Heredia P, MP; 1 m, 14 VIII 1967, LSBS, Heredia P, MP; 1 m, 15 III 1968, 4 km SW Siquirres, Limón P, BE; 2 m, 25 II 1969, LSBS, Heredia P, DP; 1 f, 21 II 1975, Finca Las Cruces, 6.5 km S San Vito de Java, Puntarenas P, DP; 2 m, 1 f, 28 III 1987, LSBS, Heredia P, BL; 3 m, 29 III 1987, LSBS, Heredia P, BL; 1 m, 1 f, 22 II 1988, LSBS, Heredia P, ES; 1 f, 30 III 1988, Upala, Alajuela P, GS; 1 m, 18 IV 1988, slope of Volcán Tenorio, Alajuela P, GS; 1 f, 6 V 1988, Colonia Libertad (attracted to light at night), Upala, Alajuela P, GL; 2 m, 1 f, VII 1988, Pitillo BS (700 m), 9 km S Santa Cecilia, Guanacaste NP, Guanacaste P, GNP; 1 f, 27 XI 1988, 10 km SW Horquetas (600 m), Puerto Viejo, Heredia P, DN; 1 f, 17 V 1989, Finca Plástico, Horquetas, Heredia P, CE; 1 m, XII 1990, Hitoy Cerere Field Station, Río Sereno (200), Hitoy Cerere BR, Limón P, CA; 1 f, 2 II 1990, near Río Dantas, BNP, Limón P, EH; 1 f, 31 III 1990, near Río Dantas, BNP, Limón P, HN; 1 f, 1 IV 1990, near Río Dantas, BNP, Limón P, HN; 1 m, III 1991, Magsasay BS, Braulio Carrillo NP, Heredia P, FE; 1 f, 13 III 1991, Magsasay BS, Braulio Carrillo NP, Heredia P, FE; 1 f, 7-26 I 1992, Hitoy Cerere Field Station, Río Sereno, Hitoy Cerere BR, Limón P, CA; 1 f, 2-9 III 1992, Pitillo BS (700), 9 km S Santa Cecilia, Guanacaste NP, Guanacaste P, MO; 1 m, 31 III – 15 IV 1992, Pitillo BS, 9 km S Santa Cecilia, Guanacaste NP, Guanacaste P, MO; 1 m, 2 f, 25-29 III 1995, Rara Avis, Horquetas de Sarapiquí, Heredia P, RA; several individuals, 24-28 V 1995, 10 km SW Horquetas, Puerto Viejo, Heredia P, DN; 3 individuals, 27 I 1998, near Río Dantas, BNP, Limón P, sighting, HG; 1 m, 8 I 1999, LSBS, Heredia P, sighting. SO; 1 specimen, 22 I 1999, near La Virgen, Sarapiquí, Heredia P, sighting. SO; 1 m, 2 f, 26 I 1999, near Río Dantas, BNP, Limón P, HE; 3 m, II 1999, near Río Dantas, BNP, Limón P, HE; 1 m, I 2000. LSBS, Heredia P, sighting. SO; 3 m, 1 f, 10 I 2000, near Río Dantas, BNP, Limón P, HE; 4 individuals, 10 I 2000 (09.05 a.m., sunny), near Río Dantas, BNP, Limón P, sighting. HE; 1 f, 19 II 2000, near Río Dantas, BNP, Limón P, HE; 1 f, 23 V 2000, near Río Dantas, BNP, Limón P, HE; 1 specimen, 5 VI 2000, near Río Dantas, BNP, Limón P, HG.
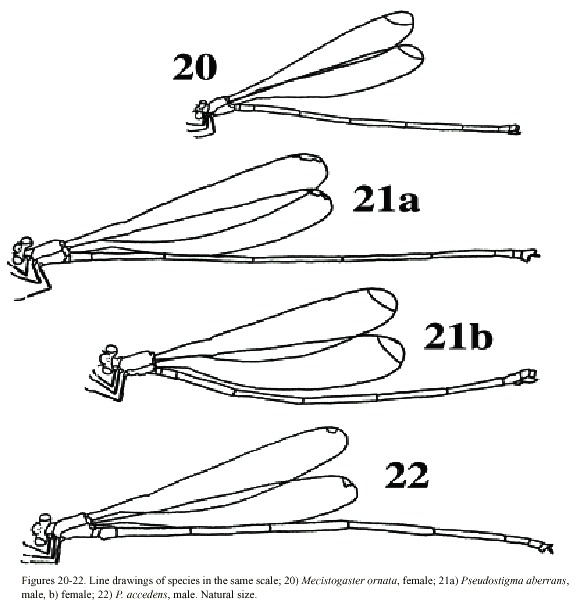
Mecistogaster ornata Rambur, 1842
Morphology: Total body size medium (Fig. 20). Colour brown or black with light markings often in a creamy hue. Wing veins brownish; paler towards wing-tips. Entire wing-tip yellowish; in females sometimes with a diffuse proximal darker area (Fig. 9); in males the membrane becomes darker ventrally when the specimens end reproductive diapause in the beginning of wet season (Fincke, 1984, 1992a, b, Westfall and May 1996). On the Pacific slope in Costa Rica, this is normally end of April-May. Such individuals may have brown wing tips ventrally, although a male from San Mateo, Alajuela Province, collected in October 1982 had dark brown wing tips also on the dorsal side. Male superior appendages evenly forcipate with the tips bent just slightly downward; light brown with darker tips. Inferior appendages short and rounded, almost not visible when viewed laterally; light or dark brown. Abdominal length in male (in mm): 67-88; female 62-86; hind wing in male 44-57; female 44-61. Measurements from specimens from southern Mexico (Westfall and May 1996) and Costa Rica.
Phenology and life zone preferences: Mecistogaster ornata especially inhabits tropical dry forest and tropical semi-dry forests, but to some extent humid lowland forests in Trinidad, southern Mexico, Central and South America down to Argentina (Beatty and Beatty 1963, Fincke 1984, Tsuda 1991). It is apparently absent from Ecuador (Tsuda 1991) but has stable populations on BCI (Fincke 1988). The species occurs in four zones in Costa Rica, from D via S (the preferred zone; X2 = 35.17, p < 0.001; Table 1) and P to an occasional finding in H (Fig. 5). It is missing entirely from the non-seasonal zone N. The findings are all located on the Pacific side of Costa Rica (Fig. 3), excluding the Nicoya Peninsula. Apparently adults can be found all year around in zones D, S and P (Fig. 5), the life cycle of the species thus well adapted to the wet and dry seasons in S. Most of the records are from October to February at the end of the wet season and first half of the dry season. We have observed M. ornata flying along human trails, stream corridors, and in sunny gaps left by tree-falls at study site 3 (Fig. 1: C; November 1997, January-February, December 1999 and January-March, May 2000). The species is easy to detect and is present in many collections, its status therefore locally common in Costa Rica, though only on the Pacific side (Fig. 3).On the Pacific slope of Volcán Orosi (550 m elevation), in north-western Costa Rica, De la Rosa and Ramírez (1995) found larvae of M. ornata living in water-filled tree-holes in a forested transitional zone between semi-decid-uous dry forest of low elevations and the mostly evergreen volcano slopes. In this area De la Rosa and Ramírez (1995) found "rain forest conditions", but with a prolonged 3 to 4 month nearly rainless dry season. Also on BCI, Fincke (1984) and Leigh and Wright (1990) found that M. ornata laid their eggs in water-filled tree-holes, in a manner similar to Megaloprepus, and that adults were seasonally abundant, emerging from November to January, during the late wet season and early dry season. Adults remained dispersed, foraging throughout the dry season. During this time of year males and females had identical yellow-tipped wings and did not react to each other when they met (Rüppell and Fincke 1989a). However in April and May, during the late dry season and early wet season, the ventral surface of a males fore wings began to darken, gradually turning black. When the male perched, only the black tips showed. Because the dorsal surface remained yellow, the sexes still appeared similar in flight (Rüppell and Fincke 1989a). In Costa Rica we have observed males with darkened wing tips in May at locality 3; see, however, the Morphology section. Roosting aggregations of M. ornata have been observed in Mexico (Beatty and Beatty 1963). Small groups of M. ornata clinged to thin vines and twiggy branches which resembled the abdomen of M. ornata in form and size. The roosting individuals of M. ornata hung on these twigs with all of the wings folded tightly together and held against the abdomen. On several occasions individuals of M. ornata were found hanging from the abdomen of another (Beatty and Beatty 1963).
Records: 1 f, 28 IX 1966, stream, 13 km WNW Esparta (60 m), Puntarenas P, MP; 1 m, 25 X 1966, Hacienda Taboga (30m), Guanacaste P, PS; 1 f, 26 X 1966, Hacienda Taboga, Guanacaste P, SE; 2 m, 17 XI 1966, Hacienda Taboga, Guanacaste P, MP; 1 f, 21 I 1967, 4 km SW Rincón de Osa, Puntarenas P, OT; 1 f, 10 II 1967, Hacienda Taboga, Guanacaste P, KI; 1 m, 11 III 1967, Rincón de Osa vic, Puntarenas P, MP; 1 m, 1 f, 15 III 1967, Rincón de Osa vic, Puntarenas P, DP; 1 m, 2 f, 29 III 1967, Brosimum site, Hacienda Taboga, Guanacaste P, MP; 3 f, 19 IV 1967, Brosimum site, Hacienda Taboga, Guanacaste P, MP; 2 f, 3 V 1967, Hacienda Taboga (30 m), Guanacaste P, DP; 1 m, 5 VI 1967, stream, 23 km WNW Esparta, Puntarenas P, DP; 1 f, 13 VII 1967, Hacienda Taboga, Guanacaste P, DP; 1 m, 5 III 1968, Road to Pacific 15 km from Rincón de Osa, Puntarenas P, BE; 2 f, 22 I 1969, Camino de Altura, Rincón de Osa, Puntarenas P, BE; 1 m, 15 I 1972, Forest edge at Monteverde (1 390 m), Puntarenas P, BU; 1 f, 7 III 1978, Llorona, Corcovado NP, Puntarenas P, BI; 1 f, 27 XII 1978, Esparza, Puntarenas P, FG; 1 f, 16 I 1980, Hatillo, San José P, SI; 1 m, 3 II 1980, Santiago de Puriscal, San José P, HI; 1 f, 20 XI 1980, Tabarcia-Mora, San José P, AR; 1 m, 1 f, 11 II 1982, Manuel Antonio NP, Puntarenas P, ES; 1 m, 25 II 1982, Manuel Antonio NP, Puntarenas P, ES; 1 f, 3 IV 1982, Corcovado NP, Puntarenas P, ES; 1 m, X 1982, San Mateo, Alajuela P, AG; 1 m, 15 X 1982, Colón, San José P, AR; 1 m, 25 November 1984,Colón, San José P, AR; 1 m, 18 VIII 1986, Las Juntas de Abangares, Guanacaste P, QU; 1 f, 1 XII 1987, Rincón de la Vieja NP (840 m), Guanacaste P, GL; 1 f, I 1989, Corcovado NP, Puntarenas P, DU; 1 m, 8 I 1989, Colón, San José P, CH; 2 f, 24 II 1989, El Rodeo Forest Preserve, near Colón, San José P, ES; 1 individual, 17-18 VII 1990, 1 km NW Golfito BR, Puntarenas P, DN; 1 m, 26 VIII 1990, Maritza BS (600 m elevation), Quebrada Tempisquito, Guanacaste NP, Guanacaste P, OB; 1 m, XII 1990, Playa Naranjo, Santa Rosa NP, Guanacaste P, AZ; 1 m, I 1991, Tacares de Grecia, Reserva Los Chorros, Alajuela P, HS; 2 individuals, III 1991, Rincón de la Vieja, Guanacaste P, JO; 1 f, 8 X 1991, Maritza BS, Santa Rosa NP, Guanacaste P, SW; 1 m, 2-19 III 1992, Pitilla BS (700 m), 9 km S Santa Cecilia, Guanacaste NP, Guanacaste P, RI; 1 f, 16 V 1992, Atenas, Alajuela P, CC; 1 m, 18 VIII 1995, near River Tárcoles, CNP, CN; 3 individuals, 7 XI 1997, near River Tárcoles, CNP, Puntarenas P, HE; 3 m, 24 I 1999, Corcovado NP (on the trail from San Pedrillo to La Llorona), Puntarenas P, sighting. SO; 2 f, 26 I 1999, Sirena, Corcovado NP, Puntarenas P, sighting. SO; 1 f, 29 XI 1999, near River Tárcoles, CNP, Puntarenas P, HE; 2 individuals, 27 I 2000, near River Tárcoles, CNP, Puntarenas P, HE; 1 individual, 10 II 2000, near River Tárcoles, CNP, Puntarenas P, HE; 2 f, 3 unidentified specimen, 8 V 2000 (11:00 a.m.; cloudy day), near River Tárcoles, CNP, Puntarenas P, HE; 1 m, 15 V 2000 (11:55 a.m.; overcast, drizzle, suspended from branch), near Río Tárcoles, CNP, Puntarenas P, HE; 1 f, 26 V 2000, near Río Tárcoles, CNP, Puntarenas P, HE; 1 f, 26 V 2000, near Río Tárcoles, CNP, Puntarenas P, HE; 1 f, 21 VI 2000, El Rodeo Forest Preserve, Colón, San José P, PO.
Pseudostigma aberrans Sélys, 1860
(Figs. 4, 10a, b, 15a, b, 21a, b)
Morphology: Total body size large, females sometimes medium (Fig. 22). Colour black, sometimes with a greenish tinge; light markings normally with a greenish colour in life (Westfall and May 1996). Wings with dark venation; lighter towards the tip. Male with a pseudostigma of 15-20 cells covering two rows (Fig. 10a) often dark, but young males probably have yellowish pseudostigmata; female with entire wing tip pale yellow; somewhat dark brown coloration proximally (Fig. 10b); sometimes inner portion of wings smoky. Abdomen in males with segment 10 expanded dorsoventrally (Fig. 15a). Male appendages yellowish with darker colour on points and ridges; superior appendages strongly forcipate, bent sharply inwards with tips pointing slightly downwards (Fig. 15a). Inferior appendages short and blunt, somewhat triangular seen laterally (Fig. 15b). Abdominal length in male (in mm): 114-130; female 85-110; hind wing in male 62-73; female 57-74.Phenology and life zone preferences: Pseudostigma aberrans is found from southern Mexico to Panama (Tsuda 1991, Paulson 1997). As far as we know P. aberrans has been seen in Costa Rica only in four occasions (see Records). P. aberrans should therefore be considered a rare species in Costa Rica (Fig. 4).
The phenology and preferences of the genus Pseudostigma is poorly known (Westfall and May 1996). The Costa Rican findings were made at localities 1, 2 and 3 (Figs. 1: B, L, C; and 4), that is in zones S and N (Fig. 5). The known adult specimens appeared in April, June and July.
In Mexico, P. aberrans was found in lowland moist forest (Westfall and May 1996). May (1979) and Fincke (1998) considered the species as an extremely rare at LSBS, and she suspected that the low abundance of the species at both LSBS and BCI, reflects some geographical range limitation, because P. aberrans as well as P. accedens (see below) are locally common in southern Mexico.
Records: 1 f, 20 VII 1988, LSBS, Puerto Viejo, Heredia P, ES; 1 f, 5 VI – 13 VII 1991, LSBS, Heredia P, sighting. FI; 1 m, 11 VII 1991, near River Tárcoles, CNP, Alajuela P, ES; 1 f, 11 IV 2000 (10.30 a.m.; sunny day), near Río Dantas, BNP, Limón P, RM.
Pseudostigma accedens Sélys, 1860
Morphology: Total body size as in the previous species (Fig. 23). The colour is brown; light areas yellowish or white. Legs dark with a yellow longitudinal stripe. Wings clear with dark venation; light towards the apex (Fig. 11; male). Male pseudostigmata as in P. aberrans. Female wing tips with a smaller yellow field than in P. aberrans. Abdomen in males with segment 10 expanded dorsoventrally (Fig. 16a). Male superior appendages sharply pointed and incurved as in the previous species. Inferior appendages small, almost not visible from the side (Fig. 16b). Abdominal length in male (in mm): 108-119; female 95-105; hind wing in male 62-68; female 60-67.
Phenology and life zone preferences: This species occurs from southern Mexico to Panama (Tsuda 1991, Paulson 1997), but so far there are no documented observations from Costa Rica, neither from Nicaragua or El Salvador.
Ramírez et al. (2000) report the species from Costa Rica; however, no details are given. Since Costa Rica and neighbouring countries are well within the known geographic range of this species, a search for it, particularly in areas not yet well investigated, may be rewarding. Nick Donnelly (pers. comm.) observed P. accedens in Guatemala and Honduras flying in two different habitats; over a temporary pond and in a somewhat open forest about 50 m. from a stream. Thus, P. accedens seems to accept a variety of habitats although at present it can not be defined to any particular zone.
Abreviations:
BR = Biological Reserve; BS = Biological Station; NA = National Park; P = Province; m = male/males; f = female/females. Collectors/ observers: AG = Aguilar R.; AN = Angulo R. M.; AO = Alvarado S.; AR = Alvarado J.; AZ = Alcazar E.; BE = Benson W. W.; BI = Bierzychudek P.; BK = Buskirk W.; BL = Belle J.; BU = Buskirk R.; CA = Carballo G.; CC = Castillo Carvajal W.; CH = Chacón I.; CN = Cannings R. A.; DN = Donnelly T. "N."; DP = Paulson D. R.; DU = Duckett C. N.; EH = Esquivel C. & Herman T.; EL = Eliazar P.; ES = Esquivel C.; FE = Fernández E.A.; FG = Figueroa F.; FI = Fincke O. M.; GL = Gonzáles L., Soto J. & Lezama H.; GN = GNP Biodiversity Survey; GP = Gustafsson, Pape & Viklund; GS = Gonzáles L. & Soto J.; HE = Hedström I.; HG = Hedström I. & Sahlén G.; HI = Hidalgo J.; HN = Herman T.; HS = Hammer Salazar A.; JO = Johansson F.; KI = Kiff L.; ; MA = Marín F.; MG = Marvin R. & Gerardo M.; MO = Moraga C.; MP = Paulson D. R. & M. L.; NA = Naumann M.; NO = Noonan G. R.; OB = Obando Arquedas, N.; OM = Orozco E., Martín F. & Ochoa R.; OT = OTS course; PO = Mario Pozla; PS = Paulson D. R. & Seifert R. P.; QU = Quesada D.; RA = Ramírez A.; RI = Ríos P.; RM = Rojas-Mendenhall A.; SE = Seifert R. P.; SI = Silva M.; SL = Stiles E. W.; SO = SaintOurs F.; ST = Starrett A.; SW = Sweeney B.; TH = Thompson F. G.; VA = Var gas R.; VK = Viklund, B.; UN = Unknown; ZU = Zumbado M. A.
Acknowledgments
Numerous data were graciously supplied by Rob Cannings, Victoria, BC, Canada; Thomas "Nick" Donnelly, Binghamton, NY, USA; Sidney W. Dunkle, Plano, TX, USA; Rosser Garrison, Azuza, CA, USA; Frank Johansson, Umeå, Sweden; Dennis Paulson, Seattle, WA, USA and Fred SaintOurs, Norwell, MA, USA. Bert Viklund, Section of Entomology, the Swedish Museum of Natural History, provided access to the old collections of Neotropical pseudostigmatids in his care. Oliver Flint, American Museum of Natural
History, Washington DC, USA supplied Pseudostigma aberrans and P. accedens for drawings. The authors gratefully acknowledges the critical review and comments of Ola Margaret Fincke, University of Oklahoma, USA, who also aided in some of the field work. The comments of three anonymous reviewers
aided us in completing the paper. We also wish to thank Paul Hanson, Universidad de Costa Rica, Carolina Godoy, Instituto Nacional de Biodiversidad (INBio), Santo Domingo de Heredia, Costa Rica and Carlos Esquivel, Universidad Nacional, Heredia, Costa Rica, for providing us access to odonate collections. We express our gratitude to the Nairi Foundation for the use of its field station near Barbilla National Park during part of our field-work. We are indebted to Javier Guevara, Ministerio de Ambiente y Energía (MINAE) in Costa Rica, for permission to carry out the investigation.
Resumen
Se presenta una clave de las especies de Pseudostigmatidae de Costa Rica. Esta familia está representada en este país por tres géneros con las siguientes especies: Megaloprepus caerulatus, Mecistogaster linearis, M. modesta, M. ornata y Pseudostigma aberrans. Se incluye también a Pseudostigma accedens, como probable en esta área. Se presenta un resumen breve de la morfología,
fenología y preferencia de zona de vida de cada especie, incluyendo mapas de distribución basados en más de 270 registros. Esto no incluye todos los ejemplares conocidos en Costa Rica pero proporciona una buena perspectiva de la distribución y estatus de cada especie. Se encontró que M. caerulatus volaba en bosque tropical seco solamente durante la primera mitad del año, pero durante todo el año en bosque tropical húmedo premontano, en bosque tropical húmedo y en bosque tropical muy húmedo, sin estación seca. Mecistogaster linearis volaba todo el año en bosque tropical húmedo y en bosque tropical muy húmedo, ambos sin estación seca. Mecistogaster modesta también volaba durante todo el año en bosque tropical muy húmedo y en bosque tropical húmedo, sin estación seca, como también en bosque tropical húmedo premontano, pero hubieron pocas observaciones de esta especie dentro del bosque tropical seco. Mecistogaster ornata estaba ausente completamente del bosque tropical muy húmedo y bosque tropical húmedo, ambos sin estación seca. Sin embargo, esta última especie volaba durante todo el año en bosque tropical seco, en bosque tropical húmedo premontano, en bosque tropical semi-seco y en bosque tropical húmedo, todos estos sin estación seca. Pseudostigma aberrans se ha observado tan pocas veces en Costa Rica que no es posible saber la preferencia del tiempo de su vuelo.
References
Beatty, G.H. & A.F. Beatty, 1963. Gregarious roosting behaviour of Mecistogaster ornata in Mexico. Proc. North Central Branch. ESA. 18: 153-155. [ Links ]
Calvert, P.P. 1901-1908. Odonata, p. 51-57. In F.D. Goldman (ed.). Biologia Centrali-Americana. Vol. 50. Insecta, Neuroptera. [ Links ]
Calvert, P.P. 1911. Studies on Costa Rican Odonata II. The habits of the plant dwelling larvae of Mecistogaster modestus. Entomol. News 22: 402-411. [ Links ]
Calvert, P.P. 1923. Studies on Costa Rican odonata X. Megaloprepus, its distribution, variation, habits and food. Entomol. News 34: 168-174. [ Links ]
Calvert, A.S. & P.P. Calvert 1917. A year of Costa Rican natural history. Macmillan, New York. [ Links ]
Carle, F.L. 1982. The wing vein homologies and phylogeny of the Odonata: A continuing debate. Societas Internationalis Odonatolgoica Rapid Communications No. 4, Utrecht. 66 p. [ Links ]
Corbet, P.S. 1999. Dragonflies: Behaviour and ecology of Odonata. Harley Books, Colchester. [ Links ]
De la Rosa, C. & A. Ramírez 1995. A note on phototactic behaviour and on phoretic associations in larvae of Mecistogaster ornata Rambur from northern Costa Rica (Zygoptera: Pseudostigmatidae). Odonatologica 24: 219-224. [ Links ]DeVries, P. 1987. The butterflies of Costa Rica and their natural history. Princeton University, Press, New Jersey. 327 p. [ Links ]
Edman, S. & I. Hedström 1999. Skogsarvet. Längs stigar i Nord och Syd. (Our ancient woodland heritage. Pathways of the North and South). Swedish National Board of Forestry, Jönköping, Sweden. 208 p. [ Links ]
Fincke, O.M. 1984. Giant damselflies in a tropical forest: Reproduction biology of Megaloprepus caerulatus with notes on Mecistogaster (Zygoptera: Pseudostigmatidae). Adv. Odonatol. 2: 13-27. [ Links ]
Fincke, O.M. 1992a. Giant damselflies of Barro Colorado Island. Behavioral Ecology. In D. Quintero & A. Aiello (eds.). Insects of Panama and Mesoamerica. Selected studies. Oxford University, Oxford. [ Links ]
Fincke, O.M. 1992b. Interspecific competition for tree holes: Consequences for mating systems and coexistence in neotropical damselflies. Amer. Natur. 139: 80-101. [ Links ]
Fincke, O.M. 1994. Population regulation of a tropical damselfly in the larval sage by food limitation, can- nibalism, intraguild predation and habitat drying. Oecologia 100:118-127. [ Links ]
Fincke, O.M. 1997. Conflict resolution in the Odonata: implications for understanding female mating patterns and female choice. Biol. J. Linn. Soc. 60: 201-220. [ Links ]
Fincke, O.M. 1998. The population ecology of Megaloprepus caerulatus and its effect on species assemblages in water-filled tree holes. In J.P. Dempster & I.F.G. McLean (eds.). Insect populations in theory and practice. Kluwer Academic, London. [ Links ]
Fincke, O.M. 1999. Organization of predator assemblages in Neotropical tree holes: Effects of abiotic factors and priority. Ecol. Entomol. 24: 13-23. [ Links ]
Förster, S. 1999. The dragonflies of Central America exclusive of Mexico and the West Indies. A guide to their identification. Odonatol. Monogr. 141 p. [ Links ]
Hedström, I. 1991. The guava fruit fly, Anastrepha striata Schiner (Tephritidae) in seasonal and non- seasonal Neotropical forest environments. Ph.D. Thesis, Uppsala University, Uppsala, Sweden. 43 p. [ Links ]
Holdridge, L.R. 1967. Life zone ecology. Tropical Science Center, San José. [ Links ]
Leigh, E.G. & S.J. Wright 1990. Barro Colorado Island and tropical biology. In A.H. Gentry (ed.). Four Neotropical rainforests. Yale University, New Haven, USA. 627 p. [ Links ]
Machado, A.B.M. & A. Martinez. 1982. Oviposition by egg-throwing in a zygopteran, Mecistogaster jocaste Hagen, 1869 (Pseudostigmatidae). Odonatologica 11: 15-22. [ Links ]
May, M.L. 1979. Lista preliminar de nombre y clave para identificar los Odonata (caballitos) de la Isla de Barro Colorado. Cuadernos de Ciencia No. 1., C.L. Castro, translator. Panama. Smithsonian Tropical Research Inst. and Editorial Universitaria. 50 p. [ Links ]
McDade, L.A., K.S. Bawa, H.A. Hespenheide & G.S. Hartshorn (eds.) 1994. La Selva: Ecology and natural history of a neotropical rain forest. Univ. of Chicago. 486 p. [ Links ]
Ramírez, A., D.R. Paulson & C. Esquivel 2000. Odonata of Costa Rica: Diversity and checklist of species. Rev. Biol. Trop. 48: 247-254. [ Links ]
Rüppell, G. & O.M. Fincke. 1989a. Mecistogaster ornata (Pseudostigmatidae). Flugverhalten und Nahrungserwerb. Foraging flight behaviour (deutsch und english). Publ. Wiss. Film. Sekt. Biol., ser. 20, Nr 7. Film E 2975. [ Links ]Rüppell, G. & O.M. Fincke. 1989b. Megaloprepus caerulatus (Pseudostigmatidae). Flugverhalten und Nahrungserwerb. Flying and reproductive behaviour (deutsch und english). Publ. Wiss. Film. Sekt. Biol., ser. 20, Nr 10. Film E 2976. [ Links ]
Paulson, D.R. 1997. Odonata of Middle America, by country. University of Puget Sound, Tacoma. [ Links ]
Tsuda, S. 1991. A distributional list of world Odonata. Osaka. [ Links ]
Valerio, C.E. 1999. Costa Rica: Ambiente y biodiversidad. Instituto Nacional de Biodiversidad (INBio), Santo Domingo de Heredia. 139 p. [ Links ]
Westfall, M.J. & M.L. May. 1996. Damselflies of North America. Scientific, Gainesville, Florida. [ Links ]
Zar, J.H. 1999. Biostatistical analysis. Prentice Hall International UK, London. [ Links ]












 uBio
uBio