Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.49 n.3-4 San José Dec. 2001
Louis M. LaPierre
Dept. Organismic Biology, Ecology, and Evolution, University of California, Los Angeles, CA, USA 90095-1606, Fax: (310) 206-3987, e-mail: louis@ucla.edu
Received 04-XI-1999. Corrected 07-111-2001. Accepted 22-111-2001.
Abstract
Cecropia is a relatively well-known and well-studied genus in the Neotropics. Methods for the successful propagation of C. obtusifolia Bertoloni. 1840 from cuttings and air layering are described, and the results of an experiment to test the effect of two auxins, naphthalene acetic acid (NAA) and indole butyric acid (IBA), on adventitious root production in cuttings are presented. In general. C. obtusifolia cuttings respond well to adventitious root production (58.3 % of cuttings survived to root), but air layering was the better method (93 % of cuttings survived to root). The concentration of auxins used resulted in an overall significantly lower quality of roots produced compared with cuttings without auxin treatment. Future experiments using Cecropia could benefit from the use of isogenic plants produced by vegetative propagation.
Key words: Costa Rica, Cecropiaceae, Cecropia obtusifolia, air layering, cuttings, vegetative propagation.
Growing plants from cuttings can be a more desirable alternative to gathering seeds or seedlings from the field for the following reasons: 1) Cuttings may be collected and propagated at any time, thus eliminating reliance on seasonally available seeds; 2) The availability of wild seedlings is patchy in both space and time; 3) Cuttings allow researchers lo done selected genotypes; 4) Clones enable researchers to test the effects of various treatments among genetically identical individuals, thus reducing the variation in intrinsic properties among individual plants. For example, plant secondary chemistry can vary considerably among sympatric conspecifics (Coley 1986 ).
Here I describe methods to propagate Cecropia obtusifolia Bertoloni, 1840 saplings from cuttings and air layering sections of the stem, and report on a preliminary experiment to determine if rooting hormones increase adventitious root production in cuttings of C. obtusifolia. Although Cecropia species fruit throughout the year, collecting seeds from ripe fruit is time consuming, and the seedlings' delicate structure require more time and care than working with cuttings and air-layered stem sections (pers. obs.). It is also noteworthy that seeds can take four weeks to germinate (Young et al. 1987).
I conducted this study at two locations: the Organization for Tropical Studies' (OTS) La Selva Biological Station (10º26' N-83°59' W; elevation: 50 - 150 m). Heredia Province, located on the Caribbean slope of the Cordillera Central at the confluence of the Río Puerto Viejo and Río Sarapiquí, and the Ecolodge San Luis and Biological Station (10º06' N-83º26' W; elevation: 1 000 - 1 300 m), Puntarenas Province, located on the Pacific slope of the Cordillera de Tilarán.
The genus Cecropia includes more than 80 species and ranges from tropical Mexico to middle South America. It is a dioecious pioneer common in disturbed areas (e.g., forest gaps, drainages, human impacted areas, etc.) from sea level to about 2 400 m elevation (Berg et al. 1990). Cecropia is involved in a well-known ant-plant mutualism in which the plants provide food for ants (primarily of the genus Azteca in Costa Rica; Longino 1991) in the form of Müllerian bodies produced by specialized tomentose pads (trichilia) located at the base of the petioles. The ants access shelter inside hollow intemodes of the stem via unique unvascularized regions (prostomata), and have been shown to reduce herbivory and the threat of encroaching vines on saplings (Janzen 1969, Schupp 1986). In the species I studied. C. obtusifolia, juvenile plants have a simple architecture consisting of a single stem and large leaves that are few in number and arranged altemately about the stem. Stem nodes on saplings are spaced between 2 and 10 cm apart.
I experimented with both cutting and air layering techniques for propagating C. obtusifolia vegetatively. For cuttings, leafIess stems from 11 saplings were cut in lengths of approximately 6 cm, 2-3 cm in diameter, such that each cutting contained at least one node. Basal resprouts of felled adult trees can also be used (pers. obs.), but were not for this study. Cuttings (n = 120) were randomly assigned a treatment or control (see following paragraph) and planted approximately 3 cm deep and 4 cm from the nearest neighbor in trays containing a rooting medium of sandy aIluvial soil, and placed inside a shadehouse. The cuttings were misted twice a day, at midday and late afternoon, to reduce stem desiccation. For air layering, ten in situ saplings were braced with a bamboo rod before their stem internodes were wounded with pruning shears at one to two node intervals, for a total of six stem sections per plant. The wounded areas were then wrapped with opaque plastic around a handful of soil. After roots appeared, I removed the stem sections from the plant and placed them in plan ter bags inside a shadehouse.
I chose the auxins naphthalene acetic acid (N AA) and indole butyric acid (IBA) for application in the cuttings experiment as they have been determined to be the most effective in initiating root formation for the majority of rooting trials reviewed by Blazich (1988). The auxins were dissolved in 50 % ethanol to formulate solutions, singly and in 50/50 combination, of 100 mg/l concentration (determined to be optimal for many plant species (Blazich 1988, Puri and Verma 1996)). A "quick dip" technique (Longman and Wilson 1993) was used to apply an equivalent amount of solution up to 2 cm above the base of each cutting. Data for the three treatrnents (NAA, IBA, and NAA+IBA) and two controls (ethanol dip and water dip) were gathered on the total number of cuttings that survived to root, on the mean number of roots produced, and the mean root length. After 14 days, the experiments were terminated and the results recorded.
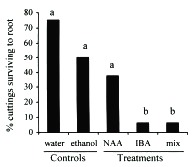
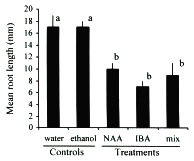
Although auxins have been determined to catalyze enzymatic reactions and thus increase the rate and quality of root production (Morsink and Smith 1974), in high concentrations they can have the opposite effect and retard or inhibit the formation of roots (Longman and Wilson 1993, Arya et al. 1994, Puri and Verma 1996). The effectiveness of auxins can also vary among species of plants and thus the optimal concentration and combination of auxins may differ among species (Haissig 1988). In general, I found the concentrations of NAA and IBA used in this experiment to result in inhibition of adventitious root production in cuttings of C. obtusifolia (Figs. 1, 2). Although the number of cuttings that survived to root after the NAA treatment was not significantly different from the controls (Fisher's exact test p > 0.05; Fig. 1), the rate at which these roots were produced, as measured by their lengths, was significantIy lower than the controls (unpaired t-test p < 0.05; Fig.2). As might be expected, the NAA+IBA mix treatment produced cuttings with intermediate values. In contrast, the cuttings exposed to the ethanol dip control experienced the same mortality and produced roots at the same rate as the water dip control, thus removing the possibility that the ethanol solution damaged the cuttings.
of the experiment. Controls are water dip and auxin-free 50 % ethanol dip.
Treatments are 100 mg/l quick dips (i.e., ethanol solutions) of the auxins naphthalene
acetic acid (NAA), indole butyric acid (IBA), and a 50/50 mix of the two. Different
letters identify significant differences at p < 0.05 (X2 and Fishers exact test).
days after the start of the experiment. Controls are water dip and auxin-free
50 % ethanol dip. Treatments are 100 mg/l quick dips (i.e., ethanol solutions)
of the auxins naphthalene acetic acid (NAA), indole butyric acid (IBA), and a
50/50 mix of the two. Different letters identify significant differences at p < 0.05
(unparied t-test).
The remaining sections produced only callous tissue along the edges of the wounds. However, seven days after these 16 sections were placed into planter bags 12 (75 %) had produced roots, the remaining sections did not survive. Thus, the overall success rate for airlayered sections that survived to root was 93 % (56 of 60). I did not collect data on number or length of roots for these stem sections.
Species of Cecropia have been the foci of studies on a wide range of questions dealing with aspects of physiology, developmental biology, and ecology. Future studies involving Cecropia where between-plant genotypic variation may be important could benefit from the use of isogenic plants propagated vegetatively. Previous studies that could have used isogenic plants include the analysis of Müllerian food body chemistry (Rickson 1976), of plant defenses against herbivores as a function of differing habitat variables (Folgarait and Davidson 1994, 1995), and of host selection among Cecropia-obligate ant species (Yu and Davidson 1997).
Acknowledgments
I am grateful to Randy Plewak and Henry Vamey at the Mildred E. Mathais Botanical Garden (University of California, Los Angeles (UCLA) for tips on vegetative propagation. Pamela Wright and an anonymous reviewer provided helpful comments on the manuscript. I thank Danilo Brenes Madrigal for editing the Resumen. The study was supported by a UCLA quarter fellowship from the Department of Organisrnic Biology, Ecology, and Evolution.
Resumen
Cecropia es un género bien conocido y bien estudiado en los Neotrópicos. Se discuten métodos exitosos para la propagación de C. obtusifolia Bertoloni, 1840 de fragmentos de troncos y acodos aéreos. A continuación se presentan los resultados de un experimento para examinar los efectos de dos tipos de hormonas (NAA e IBA) en la producción de raíces adventicias en fragmentos de troncos. En general, los fragmentos de C. abtusifalia responden bien en la producción de raíces adventicias (y sobreviven al azar 58.3% de los cortes), pero el método de acodos aéreos funcionó mejor (sobreviven al azar 93 %). El uso de hormonas resultó en raíces de baja calidad en comparación con cortes sin hormonas. Experimentos en el futuro que usan Cecropia pueden beneficiar al usar plantas isogénicas producidas por propagación vegetativa.
References
Arya, S., R. Tomar & O.P. Toky. 1994. Effect of plant age and auxin treatment on rooting response in stem cuttings of Prasapis cineraria. J. Arid Env. 27: 39-44. [ Links ]
Berg, C.C., R.W.A.P. Akkermans & E.C.H. van Heusden. 1990. Flora neotropica: Cecropiaceae: Caussapaa and Paurauma, with an introduction to the family. New York Botanical Garden, New York. 208 p. [ Links ]
Blazich, FA. 1988. Chemicals and formu1ations used to promote adventitious rooting, p. 132-149. In TD. Davis, B.E. Haissig & N. Sankhla (eds.). Adventitious root formation in cuttings. Dioscorides, Portland, Oregon. [ Links ]
Coley, P.D. 1986. Costs and benefits of defense by tannins in a neotropical tree. Oecologia 70: 238-241. [ Links ]
Folgarait, PJ. & D.W. Davidson. 1994. Antiherbivore defenses of myrmecophytic Ceerapia under different light regimes. Oikos 71: 305-320. [ Links ]
Folgarait, PJ. & DW. Davidson. 1995. Myrmecophytic Ceerapia: Antiherbivore defenses under different nutrient treatments. Oecologia 104: 189-206. [ Links ]Haissig, B.E. 1988. Future directions in adventitious rooting research, p. 303-310. In TD. Davis, B.E. Haissig & N. Sankhla (eds.). Adventitious root fonnationm cuttings. Dioscorides, Portland, Oregon. [ Links ]
Janzen, D.H. 1969. Allelopathy by myrmecophytes:The ant Azteca as an allelopathic agent of Cecropia. Ecology 50: 147-153. [ Links ]
Longino, J.T 1991. Azteca ants in Ceerapia trees: Taxonomy, colony structure, and behaviour, p. 271. 288. In R. Cutler & C. Huxley (eds.). Interactions between ants and plants. Oxford University. [ Links ]
Longman, K.A. & R. Wilson. 1993. Rooting cuttingsof tropical trees. Cornmonwealth Science Council, London. 135 p. [ Links ]
Morsink, W. & V. Smith. 1974. Root and shoot develop. ment of cuttings of basswood (Tilia americana L.) as affected by auxin treatments and size of cuttings. Can. J. For. Res. 4: 246-249. [ Links ]
Puri, S. & R.C. Verma. 1996. Vegetative propagation of Dalbergia sissaa Roxb. using softwood and hard. wood stem cuttings. J. Arid Env. 34: 235-245. [ Links ]
Rickson, FR. 1976. Anatomical development of the leaf trichilium and Müllerian bodies of Ceerapia peltata L. Amer. J. Bol. 63: 1266-1271. [ Links ]
Schupp, E.W. 1986. Azteca protection of Ceerapia: Ant occupation benefits juvenile trees. Oecologia 70: 379-385. [ Links ]
Young, K.R., U. Ewel & BJ. Brown. 1987. Seed dynarn. ics during forest succession in Costa Rica. Vegetatio 71: 157-173. [ Links ]
Yu, D.W. & D.W. Davidson. 1997. Experimental studies of species-specificity in Ceerapia-ant relationships. Ecol. Monogr. 67: 273-294. [ Links ]