Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.48 n.2-3 San José Jun. 2000
Mugilidae), in coastal areas of Northeastern Venezuela
Baumar J. Marin E.1 and Julian J. Dodson2
Received 24-II-1999. Corrected 29-II-2000. Accepted 8-III-2000.
Abstract
We studied the fecundity and growth in populations of the silver mullet, Mugil curema, in coastal areas of northeastern Venezuela between March 1992 and July 1993. The average number of ovocytes in gonads of 23-42 cm adults was 54 x 104, and the relative fecundity was 1311 ovocytes g-1 of fish. The size of mature ovocytes did not vary in different portions of the same gonad (p > 0.05) or among the adults in three populations studied. The average egg diameter for adults was 426 µm (CI= 4.34). Age of juveniles, collected from the La Restinga Lagoon at about monthly intervals, from counts of growth lines on the otoliths ranged from 50 to 240 days. The relation of age (number of growth lines) to standard length follows an exponential growth curve. The growth of juveniles varied seasonally and was greatest during the rainy season (April to August), when temperatures were highest. The time of spawning has probably been adapted so that peak recruitment into the lagoons occurs just prior to the rainy season when conditions for growth are most favorable.
Key words
Mugil curema, Mugilidae, age, growth, fecundity.
The silver mullet, Mugil curema Valenciennes 1836, a pelagic and neritic species that forms small schools along the Atlantic coast, is important in coastal fisheries in Caribbean waters. In Venezuela, it is captured in coastal seine and gill net fisheries and is most abundant during the warm water months, June and July. A number of studies have examined its growth, recruitment and reproduction (Angell 1973, Yañez-Arancibia 1978, Bustamante and Enomoto 1981, Franco 1986, Vieira 1991). It migrates offshore to spawn (Anderson 1957, Caldwell and Anderson 1959, Blaber 1987, Collins and Stender 1989, Vieira 1991, Ditty and Shaw 1996).
Several studies have described the growth of juvenile M. curema. Phillips et al. (1983) found that 50 mm individuals increased in length by 65 to 95 mm over a five months period in the Nicoya Gulf in Costa Rica. Growth increases of 115 to 130 mm during the first year have been reported for silver mullet by Alves de Araujo et al. (1980) in Brazil and by Yañez-Arancibia (1978) in Mexico. Bustamante and Enomoto (1981) reported a growth of 170 mm in 15 to 18 months in Cuba. Finally, Houde et al. (1976) observed that the growth of laboratory reared larvae over the first 36 d after hatching followed a power function and the mean daily growth increment was 0.73 mm (standard length).
Estimates of fecundity are required for studies of larval survival, in predicting stock size and in discriminating among stocks (Holden and Raitt 1974). Alvarez-Lajonchere (1982) suggested that fecundity can be used to discriminate between species and stocks of mullets and Etchevers (1974) took fecundity measures of silver mullet into account in identifying which stocks should be conserved. Etchevers (1974) observed similar fecundities for silver mullet at various sites along the northeastern Venezuelan coast, and these values were comparable to those reported by Franco (1986) for Cariaco Gulf, also in north-eastern Venezuela, and by Alvarez-Lajonchere (1982) for Cuba.
In the eastern Caribbean, the alternation between rainy and dry seasons provides a strong environmental signal in the pelagic ecosystem. During the rainy season, strong freshwater plumes develop from the Amazon and Orinoco rivers and during the dry season, wind-driven upwelling causes intensive peaks in primary production. A number of authors have discussed the contribution of land runoff and upwelling on nutrient flux in the area (Gines 1972, Gómez 1983, Ferraz-Reyes et al. 1987, Müller-Karger et al. 1989, Müller-Karger and Aparicio 1994). Studies of the temporal distribution of juvenile M. curema in coastal nursery zones are needed to understand the life history of the species and possible effects of oceanographic factors and productivity.
The recruitment of silver mullet juveniles into the La Restinga Lagoon involves two cohorts per year (B. Marin and J. Dodson, in prep.). The first is much more abundant than the second and this is related to seasonal variation in upwelling. The reproductive strategy may be an adaptation to the offshore planktonic life of silver mullet. The larval environment at the time of the pelagic phase is characterized by increased phytoplankton, abundant zooplankton prey and hydrodynamic mechanisms which should facilitate transport of young recruits to coastal lagoons (B. Marin and J. Dodson, in prep.). The presence of juveniles in the lagoon throughout the year results in the population being exposed to the seasonal changes caused by alternating rainy and dry seasons.
Silver mullet juveniles, which use the mangrove systems as a nursery area, could be strongly influenced by these factors because of their tendency to school in shallow waters (Ferrer-Montaño 1994). The objective of this study was to examine if the time when juvenile M. curema recruit into La Restinga Lagoon affects their growth as assessed by the analysis of otolith microstructure. In addition, we evaluate biological features, such as the fecundity, likely to affect reproductive capacity of adults from different fishery zones.
Materials and Methods
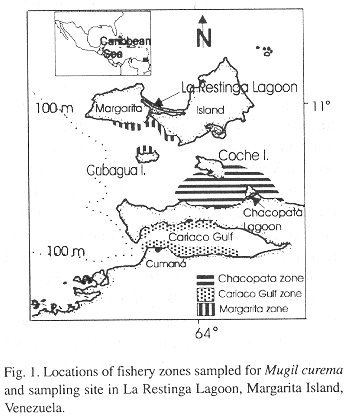
Juvenile samples. The juveniles were sampled fortnightly between March 1992 to July 1993 from La Restinga Lagoon on the southern coast of Margarita Island. The samples were captured using seine nets at three stations in the mangrove at the mouth of the lagoon (Fig. 1). Surface salinity and temperature were recorded at each sampling date.
Adult samples. Adult M. curema were sampled monthly between March 1992 to July 1993 with coastal seiners and gill nets in three commercial fishery zones, (1) the Chacopata (2) Cariaco Gulf and (3) the Margarita zones (Fig. 1).
Laboratory procedures. The juveniles collected fortnightly at each sampling zone were preserved in 95 % ethanol. For each date, we calculated a size distribution, with 4 mm size classes, based on the standard length, SL (tip of snout to hypural zone), of the fish. We observed four cohorts during the sampling period. For each cohort, a minimum of 20 individuals were randomly selected from the three most abundant size classes for otolith analysis. The otoliths (saggitae) were prepared for ageing (Stevenson and Campana 1992). Using a compound microscope, we counted the number of daily growth lines along the dorsal field of each otolith and measured its posterior radius. Three or more counts were made for each otolith. We did not use the anterior field because growth lines in this area were less consistent because of border ring losses caused by the umbrella form of otoliths. Counts made by two independent readers on eleven otoliths showed a coefficient of variation of 7.5 % (CV= 100. SD/mean) between readers (B. Marin and J. Dodson, in prep.). For the growth analysis we eliminated individuals when the coefficient of variation between replicate counts by the same reader was >10 %.
Growth Comparisons. We employed the Sigma-StatTM program (Jandel Scientific) to calculate an exponential growth curve (see Kaufmann, 1981) from the weight and length-at-age data of the first cohort produced in 1992. This curvilinear growth model, which is well suited to describing growth in young fish (Campana and Jones 1992), was as follows:
SL = a. exp [G. t]
SL = Standard length (cm)
t = Age in days (number of growth lines since the hatching mark)
G = Instantaneous growth rate
Different annual cohorts were compared based on the exponential phase of the curve. For linear regression analysis (SL-Age) of each cohort, we selected a range of sizes of juveniles between 60 and 180 mm SL. This covered the range of sizes of juveniles common to each cohort while in the lagoon. The data were fitted to a linear model prior to applying an analysis of covariance in the JMP program (SAS Institute Inc.).
For the adult fish, we visually examined the gonads to determine the sex. Fecundity was calculated in all samples from 24 females selected at advanced maturation stage, which we considered was the minimal representative number. Their gonads were extracted, measured and weighed to the nearest 0.01 g and then preserved in Gilson liquid for at least a month. Fecundity was determined by the gravimetric method. We counted the number of ovocytes under a dissecting microscope in a weighed portion of each gonad. Then we extrapolated for the total gonad based on weight (Bashirullah 1990).
For each of the 24 females we also determined the diameter of ovocytes (longest axes of each ovocyte), using a calibrated micrometer mounted in an eyepiece of a light microscope, for three subsamples of each gonad; the anterior, center and posterior portions. The subsamples were taken so that we could examine if ovocyte size was homogeneous throughout the gonad (Bashirullah 1990).
Results
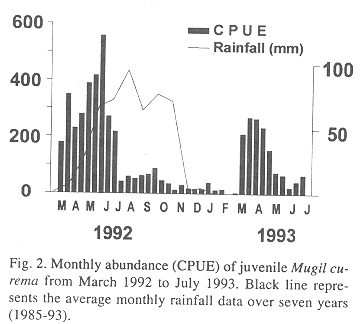
In La Restinga Lagoon juvenile silver mullet ranged from 21 to 220 mm in standard length (SL). They were most abundant at the onset of the dry season and the beginning of the rainy season. The rest of the year, they almost disappear, with only fewer and bigger individuals in the samples (Fig. 2).
Presence and growth of cohorts
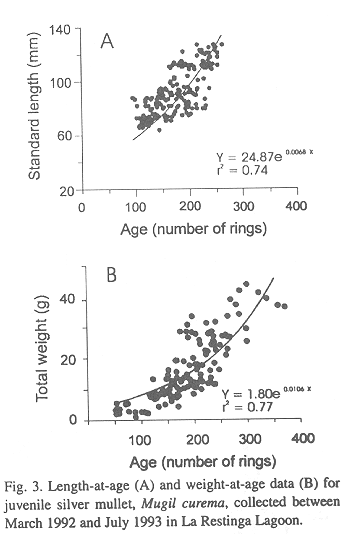
We calculated a growth curve for silver mullet based on 148 juveniles, ranging from 27 to 125 mm in length (SL in mm) and 51 to 264 days in age, which were collected between 5 March and 10 December 1992. These fish belonged to the first cohort of 1992 and represented the longest growth record of the cohorts sampled. The length to age data were best described by the following exponential curve (Fig. 3A):
SL = 24.87 e0.0068 Age
Plots of body weight (Wt in g) to age for juvenile silver mullet were also curvilinear (Fig. 3B) and were described as follows:
Wt = 1.80 e0.0103 Age
These juveniles were in the fast growing juvenile phase as observed in other fishes. Pre-adult fish (20-23 cm) were rare and only found from November 1992 to January 1993 in our samples (Fig. 4). The changes in growth seen in the growth curves were also reflected in the structure of the otoliths. The distance between lines decreased markedly as the juveniles increased in length. Further, the lines became progressively lighter with increasing age. Thus, a distinct sequence was evident.
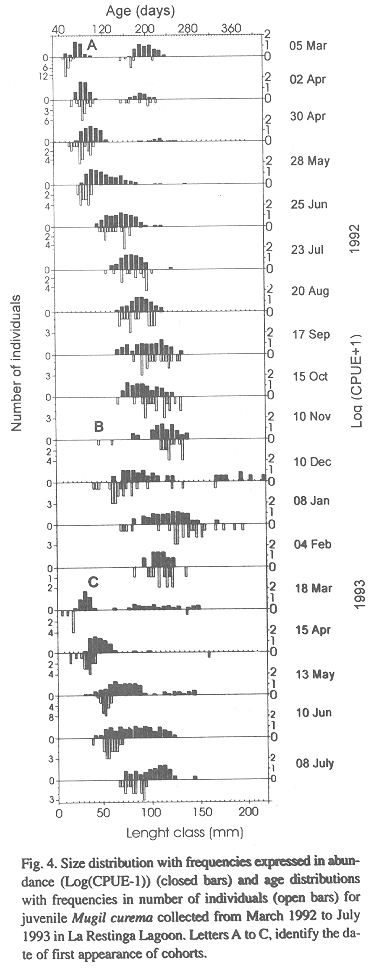
The length-frequency distributions showed the presence of two cohorts when sampling was begun on 3 March 1992, one measuring 18 to 36 mm and a second 72 to 116 in length (Fig. 4). The larger cohort disappeared within a few months, during the early rainy season (Fig. 2 and 4). The smaller cohort, which will be referred to as cohort A, was present for about 10 months, and disappeared from the samples during the dry season (December 1992). Two additional cohorts appeared during the study, cohort B, which appeared in November or December 1992, and cohort C, which appeared in March 1993. Cohort A was present in the lagoon from 25 June to 10 November 1992, cohort B from 10 November 1992 to 18 March 1993, and cohort C from 8 March to 8 July 1993.
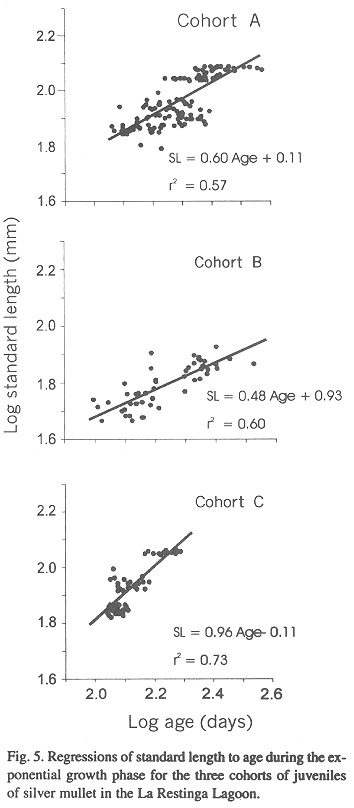
Size of the three cohorts as they entered the lagoon varied during our study (Fig. 4). Individuals of cohort A and C were similar in size and abundance whereas those in cohort B were older, larger and less abundant. Plots of age to standard length for the three cohorts over the same size range demonstrated a rapid growth rate for all three cohorts (Fig. 5) but an analysis of covariance showed that rates varied among cohorts (F = 7.848, p < 0.001, Table I). The best growth was for cohort C (Fig. 5).
Statistics of ANCOVA of the linear function of growth
(log LS versus logAge) for the three cohorts sampled.
| Source of relation | | | |
| | | | |
| A x B x C | | | |
| A versus B | | | |
| A versus C | | | |
| C versus B | | | |
A: first cohort 1992, B: second cohort 1992 and C: first cohort 1993.
Size at maturity
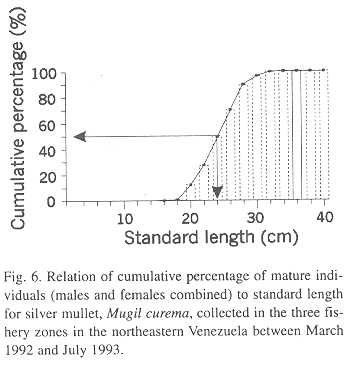
A curve of cumulative percentage of mature individuals to standard length showed that the threshold of 50 % gonadal maturity was attained at 24 cm (Fig. 6).
Fecundity and ovocyte diameter
Fecundity estimates ranged from 19 x 104 to 110 x 104 ovocytes per individual and the average of the 24 adults measuring 23 cm to 42 cm in length was 54 x 104 ovocytes per individual. The relationship of fecundity to standard length (SL, cm) and body weight (Wt, g) were are follows:
| | |
| | |
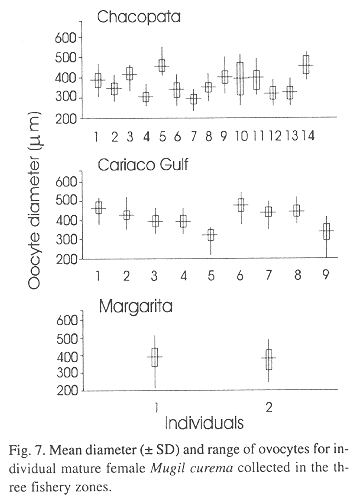
The mean diameter of ovocytes in the developed gonads for the fish in the various samples varied from 200 to 550 mm (Fig. 7). There were not significant differences between diameters of ovocytes of three parts of the same gonads (F = 3.07, p > 0.05). Hydrated ovocytes were not observed.
Environmental data
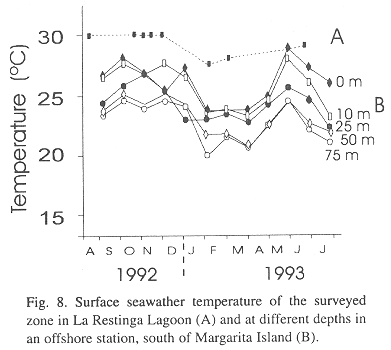
Sea surface temperatures inside the lagoon was quite stable, 27 to 32° C, with an average of 29° C (Fig. 8). The warmer surface temperatures from May to November 1992 corresponded to the rainy season (Fig. 2) and colder temperatures from December 1992 to March 1993 to the dry season. Surface seawater temperatures in the lagoon were higher than in the neighboring offshore Margarita zone (Fig. 8).
Discussion
Our estimates of fecundity are high compared to those reported for M. curema in the previous study by Angell (1973) in La Restinga Lagoon, in the study by Franco (1986) in Cariaco Gulf and in a study by Alvarez-Lajonchere (1982) in Cuba. Further they are greater than those reported for other mugilids (Alvarez-Lajonchere (1982), Salem and Mohammad 1983, Render et al. 1995). The high variability in fecundity for this species (20 to 100 x 104 ovocytes per fish) means that, contrary to Etchevers (1974), fecundity is not likely to be useful in discriminating between stocks.
Backcalculation of birth dates, calculated from growth line counts on the otolith, demonstrated that the cohort A found in March 1992, was produced from spawning between late December 1991 and late March 1992 (B. Marin and J. Dodson, in prep.). This cohort was present throughout 1992 and disappeared in early 1993. The backcalculated birth date of cohort B, which appeared in the lagoon between November 1992 and January 1993, was July to August 1992. It dominated the population until February 1993. Finally, birth date of cohort C, which appeared in March 1993, was January to February 1993. Residency inside the lagoon was longer for cohorts A and C, which were smaller in size and produced during upwelling, than for cohort B, which was not produced during upwelling and was much larger when it entered to the lagoon.
The recruitment of juvenile M. curema into La Restinga Lagoon was more protracted than that observed by Anderson (1957) into coastal areas in Florida and by Vieira (1991) into coastal areas of Brazil. Anderson (1957) and Jacot (1920) reported juveniles as small as 17 to 20 mm in coastal areas and erratic and low recruitment up until August. Yanez-Arancibia (1978) observed schools of juveniles measuring 50 to 70 mm enter the lagoons on the Pacific coastal lagoons of Mexico in August. The periods and sizes of cohort A and C were similar but we found bigger sizes in the second recruitment of the year (cohort B) (Fig. 4). The smaller recruitment size for cohorts A and C (17 to 30 mm) suggests a shorter demersal period on the shelf before entering La Restinga Lagoon.
The size of juveniles recruiting into lagoons between 12 mm (maximal larval size of M. curema captured at offshore stations, Marin personal observation) to 17 mm (minimal captured juvenile size at coastal stations), corresponds to the expected size of postlarvae and early juveniles from the growth equation. These events of fish development are reflected in otolith structure. Discontinuities in growth lines usually reflect marked ontogenic changes such as the change from larval to juvenile phases (Balon 1984). Growth lines in juvenile M. curema in La Restinga Lagoon are formed in a surprisingly regular pattern, considering the strong variations in this environment. The temperatures in the lagoon (28 to 32°C) are near the optimal levels reported by Ciurcina and Chung (1983) for M. curema and well below its upper lethal limit (37°C). This may explain its abundance in estuarine lagoons (Yañez-Arancibia 1976).
Coastal lagoons are excellent natural areas for juvenile fishes because of the turbidity which makes them less vulnerable to visual predators. Further, food is abundant for detritivorous species. They are considered one of the main nursery areas supplying marine trophic foodwebs (Caddy and Sharp 1986). Rainy periods in estuarine zones and lagoons offer both increased primary production and an increased abundance of detrital matter. The use of coastal mangrove lagoons as nursery areas by M. curema during their rapid-growing juvenile phase is likely a good survival strategy. The greater growth observed for the cohort C (Fig. 5) suggested better conditions in the lagoon for juveniles during the April-July period when the density of mullet was low. The density of recruits has been proposed to explain density-dependent mortality related to the carrying capacity of temperate coastal nursery areas (van der Veer 1986, Koutsikopoulos et al. 1991). The difference between cohort C and cohort A may be explained by differences in growth due to density dependent effects (e.g.Jordan 1993).
Warmer water temperatures are associated with the rainy period. Gómez (1983) reported that temperature variations in La Restinga Lagoon are more stable (around 2°C) than in offshore areas and that the warmest period is between June and November. During the warm period chlorophyll a levels are highest and much of the primary production is exported to adjacent coastal zones (Gómez 1983). Increased production of phytoplankton during the warmer months has also been reported for other lagoons in the region (Ferraz-Reyes et al. 1987). We observed similar seasonal temperature pattern (Fig. 8) and we believe that this period offers the best food supply for juveniles according to the results of these previous studies. It would appear that the timing of the spawning period of M. curema has been selected so that peak recruitment of juveniles occurs in the pre-rainy period (April-May). This allows the juveniles to take full advantage of the rainy season in the lagoons when food and temperature conditions are most favorable.
The evident variability in food and temperature conditions are likely to affect the growth of juveniles (Suthers et al. 1989, Jenkins et al. 1991, Jordan 1994). When food is abundant, an increase in temperature will lead to maximal growth, up until the optimum temperature for growth is exceeded (Jobling 1994). The increased growth of the low density cohort C (Fig. 5) during the warm period (Gómez 1983) suggests that trophic conditions are critical for growth. Accelerated growth is likely advantageous in that it should increase the ability of fish to escape predators and thus attain sexual maturity.
During the period when the juveniles have exponential growth, they form schools and are benthic feeders. These schools are abundant in mangrove lagoons in the Caribbean region and are a major food source for higher trophic levels (Yañez-Arancibia 1976). At 190-230 mm in size, mullet begin to leave the lagoons. The maximal size in the lagoons (Fig. 4) indicates the size at which they migrate to offshore areas for reproduction. At this size more energy is channeled into the gonads and the logistic growth phase begins. This size corresponds to the minimal size in commercial captures taken offshore from La Restinga Lagoon. It is thus the recruitment size for the commercial fishery. The importance of mangrove nursery areas on the Venezuelan coast for commercial fish clearly indicates the need to protect and conserve these ecosystems.
Acknowledgments
This work is part of a a Ph.D. thesis submitted to Laval University financed by a Fundayacucho Program of Scholarships. We thank the technical and analytical assistance of the zooplankton staff of IOV-UDO: Domingo Figueroa, Rafael Briceño, Thays Allen and Santiago Méndez. We thank Caroline Berger for her work in field survey, figures and otoliths processing aside Julie Paquet. Mr. Martin Llano, of the Metereological Station of Fundacion La Salle, kindly supplied the environmental data. The support and advice of John Himmelman, John Bonardelli and Cesar Lodeiros, were very helpful. The research was funded by GIROQ (Dep. Biologie, Universite Laval) and Consejo de Investigacion-Universidad de Oriente, proyecto LISA-92 (CI-5-019-00554/92).
References
Alvarez-Lajonchere, L. 1982. The fecundity of mullet (Pisces, Mugilidae) from Cuban waters. J. Fish Biol. 21: 607-613. [ Links ]
Alves de Araujo, R., S. Ramanathan & N. Tiruvenkatachary. 1980. Prospects of brackish-water fish culture (Mugilidae) in the state Rio Grande do Norte, Brasil. Cienc. Cult. Com. 32: 1510-1513. [ Links ]
Anderson, W. W. 1957. Early development, spawning, growth and occurrence of the Silver Mullet (Mugil curema) along the south Atlantic Coast of the United States. US Nat. Mar. Fish. Serv. Fish. Bull. 57: 415-425. [ Links ]
Angell, Ch. 1973. Algunos aspectos de la biología de la lisa, Mugil curema Valenciennes, en aguas hipersalinas del nororiente de Venezuela. Mems. Soc La Salle Cienc. Nat. 51: 223-238. [ Links ]
Balon, E. K. 1984. Reflections on some decisive events in the early life of fishes. Trans. Am. Fish. Soc. 113: 178-185. [ Links ]
Bashirullah, A.K.M. 1990. Reproductive biology of Spanish Makerel Somberomorus brasiliensis Collette, Russo & Zavala-Camin 1978 (Pisces: Scombridae) in Eastern Venezuela. Bol. Inst. Oceanogr., Univ. Oriente 29: 91-96. [ Links ]
Blaber, S.J.M. 1987. Factor influencing recruitment and survival of mugilids in estuaries and coastal waters of Southeastern Africa. pp. 57-66. In: M. Dadswell, R. Klauda, C. Saunders, R. Rulifson and J. Cooper (eds.). Common strategies of anadromous and catadromus fishes. Proceedings of a International Symposium. Boston. Massachusetts, USA. March 9-13, 1986. [ Links ]
Bustamante, G. & Y. Enomoto. 1981. Cultivo experimental de Lisas (Mugil curema, M. liza y M. trichodon) en estanques. Inf. Cienc. Tec. Inst. Oceanol., Acad. Cienc., Cuba. 158: 1-23. [ Links ]
Caddy, J. F. & D. Sharp. 1986. An ecological framework for marine fishery investigation. FAO Fish. Tech. Pap. 182: 1-152. [ Links ]
Caldwell, D. K. & W. W. Anderson. 1959. Offshore occurrence of larval silver mullet, Mugil curema, in the western Gulf of Mexico. Copeia 1959: 252. [ Links ]
Campana, S. E. & C. M. Jones. 1992. Analysis of otolith microstructure data. pp. 73-100. In: Otolith microstructure examination and analysis. D.K. Stevenson & S.E. Campana (eds.). Can. Spec. Publ. Fish. Aquat. Sci. 117: 1-126. [ Links ]
Campana, S. E. & J. D. Nielsen. 1985. Microstructure of fish otoliths. Can. J. Fish. Aquat. Sci. 42: 1014-1032. [ Links ]
Ciurcina, P. & K. S. Chung. 1983. Efectos de la temperatura ambiental y la temperatura de aclimatación sobre la tolerancia térmica en ejemplares juveniles de lisa Mugil curema., Bol. Inst. Oceanogr., Univ. Oriente 22: 35-42. [ Links ]
Collins, M.R. & B.W. Stender. 1989. Larval stripped mullet (Mugil cephalus) and white mullet (M. curema) off the southeastern United States. Bull. Mar. Sci. 45: 580-589. [ Links ]
Ditty, J.G & R.F. Shaw. 1996. Spatial and temporal distribution of larval stripped mullet (Mugil cephalus) and white mullet (M. curema, Family: Mugilidae) in the Northern Gulf of Mexico, with notes on mountain mullet, Agonostomus monticola. Bull. Mar. Sci. 59: 271-288. [ Links ]
Etchevers, S. L. 1974. Fecundidad de la Lisa (Mugil curema Valenciennes) en el Oriente de Venezuela. Bol. Cient. Téc., Ser. Rec. Mar., CIC.UDO. 1: 1-19. [ Links ]
Ferraz-Reyes, E., E. Mandelli y G. Reyes-Vasquez. 1987. Fitoplancton de la Laguna Grande del Obispo, Venezuela. Bol. Inst. Oceanogr., Univ. Oriente 26: 111-124. [ Links ]
Ferrer-Montaño, O. J. 1994. Recruitment of White Mullet in Lake Maracaibo, Venezuela. North Am. J. Fish. Manag. 14: 516-521. [ Links ]
Franco, L. 1986. Biología y reproducción de la lisa, Mugil curema Valenciennes (Pisces: Mugilidae) en el Golfo de Cariaco, Venezuela. Tesis de Maestría. Instituto Oceanográfico de Venezuela, UDO, 103 p. [ Links ]
Gines, H. 1972. Cartas Pesqueras de Venezuela. Fundación La Salle de Ciencias Naturales. Caracas. 341 p. [ Links ]
Gómez, A. 1983. Pigmentos clorofílicos, producción primaria y abundancia planctónica en el canal de entrada a la laguna de La Restinga, Venezuela. Bol. Inst. Oceanogr. Univ. Oriente 22: 43-63. [ Links ]
Holden, M.J. & D.F.S. Raitt. 1974. Manual of Fisheries science. Part 2- Methods of resource investigation and their application. F.A.O. Fish Tech. Pap. 115, Rev. 1: 1-214. [ Links ]
Houde, E. D., S. A. Berkeley, J. J. Klinovsky & R. C. Schekter. 1976. Culture of larvae of the white mullet, Mugil curema Valenciennes. Aquaculture 8: 365-370. [ Links ]
Jacot, A. P. 1920. Age, growth and scale characteristics of the mullets, Mugil cephalus and Mugil curema. Trans. Am. Microsc. Soc. 39(3): 119- 229. [ Links ]
Jenkins, G. P., J. W. Young & T. L. O. Davis. 1991. Density dependence of larval growth of a marine fish, the southern bluefish tuna, Thunnus maccoyii. Can. J. Fish. Aquat. Sci. 48: 1358-63. [ Links ]
Jobling, M. 1994. Fish Bioenergetics. Fish and Fisheries Series 13. Pub. Chapman and Hall. Londres. 309 p. [ Links ]
Jordan, A. R. 1993. Age, growth and back-calculated birthdate distribution of larval Jack Mackerel, Trachurus declives (Pisces: Carangidae) from Eastern Tasmanian coastal waters. Aust. J. Mar. Freshw. Res. 45: 19-33. [ Links ]
Kauffman, K. W. 1981. Fitting and using growth curves. Oecologia 49: 293-299. [ Links ]
Koutsikiopoulos, C., L. Fortier & J. A. Gagné. 1991. Cross-shelf dispersal of Dove sole eggs and larvae (Solea solea) in Biscay Bay and recruitment to inshore nurseries. J. Plankton Res. 13: 923-946. [ Links ]
Müller- Karger, F. E. & R. Aparicio C. 1994. Mesoscale processes affecting phytoplankton abundance in the southern Caribbean Sea. Cont. Shelf Res. 14: 199-221. [ Links ]
Müller- Karger, F. E., C. R. McClain, T. R. Fisher, W. E. Esaias & R. Varela. 1989. Pigment distribution in the Caribbean Sea: Observations from space. Prog. Oceanogr. 23: 23-64. [ Links ]
Phillips, P. C., I. Astorga, C. Hidalgo & A. Villarreal. 1983. Daily and monthly variation in abundance, diversity and composition of littoral fish populations in the Gulf of Nicoya, Costa Rica. Rev. Biol. Trop. 31: 297-306. [ Links ]
Render, J. H., B. A. Thompson & R. L. Allen. 1995. Reproductive development of Stripped Mullet in Louisiana estuarine waters with notes on the applicability of reproductive assessment methods for isochronal species. Trans. Am. Fish. Soc. 124: 26-36. [ Links ]
Salem, S. A. & S. Z. Mohammad. 1983. Studies on Mugil semeli and Mugil capito in Lake Timsah. I. Age and growth. Bull. Inst. Oceanogr. Fish., ARE 8: 31-64. [ Links ]
Stevenson, D. K. & S. E. Campana. 1992. Otolith microstructure examination and analysis. Can. J. Fish. Aquat. Sci. 117: 1-126. [ Links ]
Suthers, Y. M., K. T. Frank & S. E. Campana. 1989. Spatial comparison of recent growth in postlarval Atlantic cod (Gadus morhua) off sutherwestern Nova Scotia: inferior growth in a presumed nursery area. Can. J. Fish. Aquat. Sci. 46: 113-124. [ Links ]
Van der Veer, H. W. 1986. Immigration, settlement, and density-dependent mortality of a larval and early post-larval 0-group plaice (Pleuronectes platessa) population in the western Wadden Sea. Mar. Ecol. Prog. Ser. 29: 223-236. [ Links ]
Vieira, J. P. 1991. Juvenile mullets (Pisces: Mugilidae) in the Estuary Lagoa dos Patos, RS, Brazil. Copeia 1991: 409-418. [ Links ]
Warlen, S. M. 1988. Age and growth of larval Gulf Menhaden, Brevoortia patronus, in the northern Gulf of Mexico. US Nat. Mar. Fish. Serv. Fish. Bull. 86: 77-90. [ Links ]
Yánez-Arancibia, A. 1976. Observaciones sobre Mugil curema Valenciennes en áreas naturales de crianza, México. Alimentación, crecimiento, madurez y relaciones ecológicas. An. Centro Cienc. del Mar y Limnol., Univ. Nac. Autón. México 3: 92-124. [ Links ]
Yánez-Arancibia, A. 1978. Taxonomía, ecología y estructura de las comunidades de peces de lagunas costeras con bocas efímeras del Pacífico de México. Centro Cienc. del Mar y Limnol., Univ. Nac. Autón. México, Publ. Esp. 2: 1-306. [ Links ]
1 Instituto Oceanográfico de Venezuela. Universidad de Oriente. Cumaná 6101. Venezuela. Fax: 93-512276, e-mail: bmarin@cumana.sucre.udo.edu.ve
2 Contribution to the program GIROQ (Groupe Interuniversitire de Recherche Océanographique du Québec). Départément de biologie. Université Laval. Québec G1K 7P4. Québec. Canada. Fax: 656-2043, e-mail: julian.dodson@bio.ulaval.ca