Revista de Biología Tropical
versión On-line ISSN 0034-7744versión impresa ISSN 0034-7744
Rev. biol. trop vol.48 no.1 San José mar. 2000
Fusarium equiseti) of cotton (Gossypium hirsutum) in Adamawa in Nigeria
I. B.Chimbekujwo. 1
Cotton fungi were surveyed in Ngurore, Adamawa, Nigeria in 1992 and 1993 by counting the number of isolates in each 100 infested plants per plot. Approximately 90% of the isolated fungi were Fusariumsolani and Fusarium equiseti, both pathogenic; F. solani isolates were more virulent and frequent than F. equiseti. The high frequency and virulence of both fungi make them important pathogens of cotton in the area.
Key words
Cotton, Fungi, Fusarium, Gossypium, ecology.
Upland cotton (Gossypium. hirsutum L.) is an important cash crop and food in the world (Prentice 1972). Seedling disease in cotton is a worldwide problem, particularly the wilt and root rot disease causing loss to farmers (Hillocks 1992). The most common fungi associated with cotton disease are Fusarium spp., Colletotrichum gossippi, Rhizopus spp., Thielavispsisbasicola and Pythium (King and Presley 1942; Roy and Bourland 1982; Johnson et al. 1978, Mauk and Hine 1988 and Hillocks 1992).
Materials and Methods
Disease survey: Systematic disease surveys were conducted in the cotton growing plots between May and July 1992 and 1993. The number of isolates in each 100 infested plants from two plots were counted and expressed in percent (%).
Results
Two fungi isolated were identified to be F. solani (Mart.) Sacc. Teleomorph; Nectria haematococcca and F. equiseti (Corda)Sacc. The two fungal isolates were confirmed by IMI to be the same organisms with the number IMI 368692 and IMI 368693 respectively.
TABLE 1
Prevalence of cotton seedling wilts given in terms of Fusarium isolates (%)
| Year | | | ||
| Plot N° | | | | |
| F. solani | | | | |
| F. equiseti | | | | |
| Others | | | | |
TABLE 2
Growth characteristics of the two isolates on PDA in five days at 30°C ± 1.
| | | |
| | Mycelia white. Back of plate yellowish pigments. | Mycelia cottony white. Back plate white. |
| | Mycelia spread very fast. Spores produced. Yellow pigmentation increased. Zonation. Hyphae branched and septate | Mycelia grew slowly. Macrospores produced. Violet pigments. Zonation. Hyphae septate |
| | Three zonations. Pigmentation increased. Sparse mycelia. | Woolly mycelium. Pink colour increased. |
| | Zones increased. Mycelia scanty and withered. Colour pale. Back yellowish. | Radial growth. Zonation not clear. Mycelia yellowish. |
| | | | |||
| | | | | | |
| | | | | | |
| | | | | |
|
TABLE 4
The dry weight (g)of mycelia and pH of the two fungi for 30 days incubation grown
in a liquid medium of potato dextrose at 25°C ± 1.
|
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
Discussion
The role of Fusarium spp. as a pathogen of cotton seedlings, and other crops is well known. But Johnson and Doyle (1986) reported that Fusarium spp. were not important pathogens in cotton seedling disease complex, even though Fusarium spp. were the most frequently isolated fungi. The species involved were not identified by them.
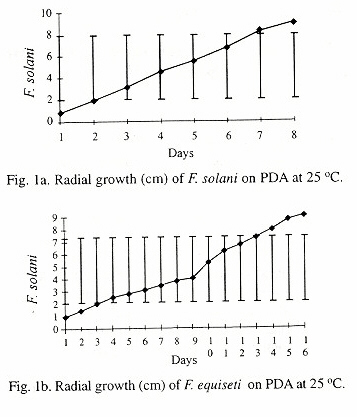
The observed morphological and cultural characteristics of F. solani and F. equiseti was the same as that reported by Booth (1971) and Joffe (1986), except that the growth rate differed. The growth rates of F. solani and F. equiseti were 3.2cm and 5.8cm, while the average growth rates observed were 1.3cm and 0.3cm for the two fungi, respectively. The differences in the growth rates may be due to the growth medium and the incubation condition.
References
Abbas, H.K., T Tanako & S. O.Duke. 1995. Pathogenicity of Alternariaalternata and Fusarium moniliforme and pathogenicity of AA1-toxin and Fumonism B1 tomato cultivars. J. Phytopathol. 143: 329-334. [ Links ]
Ben-Yephet, Y. M. Reuven & A. Genizi. 1994. Effects of inoculum depth and density on Fusarium wilt in carnations. Phytopathology 84: 1393-1398. [ Links ]
Bollenbacker, K. & N.D. Fulton. 1970. Disease susceptibility of cotton seedling from artificially deteriorated seeds. Pl. Dis. Rep. 59: 222-227. [ Links ]
Booth, C.1971. The genus Fusarium. CAB, CMI. England.46-49, 157-159p [ Links ]
Colyer, P.D. 1988. Frequency and pathogenicity of Fusarium spp. associated with seeding diseases of cotton in Louisiana. Pl. Dis. 72: 400-402. [ Links ]
Dunlap, A.A. 1941. A convient soil culture method for obtaining sclerotia of the cotton root rot fungus. Amer. J. Bot. 28: 945-947. [ Links ]
Haware, M.P. & Y.L. Nene. 1982. Races of Fusarium oxysporum f.sp. cerci. Pl. Dis. 66: 809-810. [ Links ]
Kappelman A.J.JR. 1975. Fusarium wilt resistance in cotton (Gossypiumhirsutum). Pl. Dis. Rep. 59: 803-805. [ Links ]
Kappelman A.J.JR. 1982. Resistance to Fusarium wilt pathogen in currently used cultivars. Pl. Dis. 66: 837-839. [ Links ]
Katan, J. 1971. Symptomless carriers of the tomato Fusarium wilt Pathogens Phytopathology 61: 1213-1217. [ Links ]
Katan, T. & J. Katan. 1988. Vegetative-compatibility grouping of, Fusariumoxysporum f.sp. vasinfectum from tissues and the rhizosphere of cotton plants. Phytopathology 78: 852- 855. [ Links ]
Kerbabaeva A.A. & I.P. Frolov. 1986. Fungi of the genus Fusarium isolated from cotton of Tashauz Oblast Turkmen-SSR USSR: IZV Akad Nauk. Turkm SSR SER BIOL NAUK. 6: 60-61
King, C.J. & C Presley. 1942. A root rot of cotton caused by Thielopviopsisbasicola. Phytopathology 32: 752-761. [ Links ]
Klich M. 1986. Mycoflora of cotton seed from the southern USA a three year study of distribution and frequency. Mycology 78: 706-712 [ Links ]
Lekwa, G. & E.K. Nto. 1982. Cotton soil of Nigeria and their management-proceeding of the first National symposium on cotton production, Samaru-Zaria. Pg. 120-130. [ Links ]
Manandhar, J.B., G.L. Hartman & T. C. Wang. 1995. Conidial germination and appressorial formation of Collectotrichum capsici and C. gloeosporioides isolates from pepper. Pl. Dis. 79:361-366. [ Links ]
Mauk, P.A. & R.B. Hine. 1988. Infection, colonization of Gossypiumhirsutum and G. barbadense and development of black root rot caused by Thielaviopsis basicola. Phytopathology 78: 1662-1667. [ Links ]
Melero-Vara J.M. & R.M. Jimenez-diaz. 1990. Etiology incidence and distribution of cotton seedling damping-off in southern Spain. Pl. Dis. 74: 597-600 [ Links ]
Minton, B.F. & R.H. Garber. 1983. Controlling the seedling disease complex of cotton. Pl. Dis. 67: 115-118. [ Links ]
Mousa E.M., A.A. Gaafar & El-Shennawy 1990. The influence of root-knot nematode on damping-off and wilt fungi of cotton. Nematology 36: 373 [ Links ]
Nelson, P.E., T.A. Toussoun & R.J. Cook. 1981. Fusarium : Disease, Biology and Taxonomy. Pennsylvania state University, United State of America 29-38p. [ Links ]
Parkinson, D., T.R.G. Gray. & S.T. Williams. 1971. Methods for studying the ecology of soil microbiology organisms. 1-16p. [ Links ]
Pizzinatto M.A. & J. O.M. Menten. 1991. Pathogenicity of eight Fusarium spp isolated from seeds to cotton seedlings. Sum. Phytopathology 17: 124-134 [ Links ]
Prentice, A.N. 1972. Cotton: with special reference to Africa. Pub. Ltd. London.. 1-20p. [ Links ]
Ray, W.W. & E. Mclaughline. 1942. Isolation and infection tests with seed and soil borne cotton pathogens. Phytopathology 32:233-238. [ Links ]
Roy, K.W. & F.M Bourland. 1982. Epidemiological and relationships in cotton seedling disease in Mississippi, Phytopathology 72:868-872. [ Links ]
Scherger, A.C. & D.J Mitchell. 1993. Influence of mucilage secreted by macroconidia of, Fusarium solani f.sp. phaseoli on spore attachment to roots of vigna radiata in hydroponics nutrient solution. Phytopathology 83: 1162 -1170. [ Links ]
Sharma Y.R. & B.S. Sandhu. 1986. A new fungus associated with boll rot of arboreum cotton. Cur. Sci. (Bangal).54: 937 [ Links ]
Simpson, M.E., P.B. Marshi, G.V. Merola. R.J. Ferretti. & E.C. Filsinger. 1973. Fungi that infect cotton seeds before harvest. Appl. Microbiol. 26: 608-613. [ Links ]
Soleymani, M.J., G.A. Hedjaroude. & J. Zad. 1993. Survey on mycoflora of cotton seed in Iran. Iran. J. of Pl. Pathol. 29: 55-56 [ Links ]
Soleymani, M.J., G.A. Hedjaroude. & J. Zad. 1993. Studies on pathogenicity of some seedborn Fusarium species on cotton seedling. Iran. J.of Pl. Pathol. 29:19-20. [ Links ]
Sparnicht, R.H. & R.W. Roncardori. 1972. Fusarium boll rot of cotton: pathogenicity and histopathology. Phytopathology 62: 1381-1386. [ Links ]
Watkins, G.M. 1981. Compendium of cotton disease. Pub. By the American Phytopathology society. 1-87p. [ Links ]
Weindling, R., P.R. Miller. & A.J. Uiistrup. 1941. Fungi associated with disease of cotton seedlings and bolls, with special consideration of Glomerellagosypii. Phytopathology 31: 158-167. [ Links ]
Woodroof, N.C. 1927. A disease of roots produced by, Fusarium moniliforme sheld. Phytopathology 17: 227-238. [ Links ]












 uBio
uBio