Revista de Biología Tropical
versión On-line ISSN 0034-7744versión impresa ISSN 0034-7744
Rev. biol. trop vol.47 no.4 San José dic. 1999
Mochokidae) in Asa lake, Ilorin, Nigeria
P. A. Araoye 1
Received 13-VIII-1998. Corrected 2-VI-1999. Accepted 9-VI-1999.
Abstract
Spatio-temporal distribution of Synodontis schall in Asa lake was studied for 24 months (March 1991 to February 1993). Distribution of individual was: 28.40 % (surface), 35.60 % (shore), and 36.0 % (bottom). Catches within the habitat were not significantly different. Similarly catches within the habitats during the periods of wet (May to October) and dry (November to April) seasons were not significantly different. There was seasonal occurrence of the fish at the bottom because the catches from this habitat in October (4.80 to 9.10 %) and November (0.00 to 8.70 %), corresponding with the period of flood and high water levels, were relatively low due to feeding and reproductive phenomena. Although catch was inversely proportional to the water levels, this was not significant in this experimental gill net catches. Synodontis schall was caught throughout the sampling period indicating its successful adaptation within the environment due to low predation and its diverse feeding habits.
Key words
Synodontis schall, surface, shore, bottom habitats.
The family Mochokidae consists of three genera, Synodontis, Chiloglanis and Mochokus, of which the genus Synodontis is the most common and of great commercial importance (Reed et al. 1967). Synodontis species only occur in Africa and, apart from those species present in the River Nile, they are restricted to water systems within the tropics (Willoughby 1974, Berra 1981).
Some early work on this genus includes the food and feeding habits by Pekkola [1919]. Further work on this was reported by Sandon and El-Tayed [1953] and studies on fecundity were made by Nawar (1958, 1959). Recent report on this genus in Nigeria includes that of Oni et al. (1983) on comparative physiology, Olatunde (1989) on the Biology of S. schall in Zaria and Araoye (1997) on Ecology of the Mochokid in Asa lake. Work done on distribution and abundance was restricted to very large water bodies, including Lake Volta, the Upper Nile, Lake Chad and Kainji Lake of Nigeria.
In the Nile, total catch from the commercial fisheries was reported to increase with increase in water levels (Bishai and Abu Gideiri 1967).
Twenty-one species have been reported to occur in Nigerian inland waters and most of them were found within the Sudanean and Guinean zones while very few species, like S. schall occur universally (McConnell 1965, Reed et al. 1967). Ita (1978) observed in Kainji Lake that S. membranaceus and S. resupinatus exhibited distinct habitat preferences. The former prefers unclear areas while the latter prefers habitat with close proximity to the shoreline and fairly deep waters.
This works which is the first scientific report on S. schall in the lake, is aimed at providing information on the spatial and temporal distribution of the fish to enlighten artisanal fisheries on where and when to set their nets around the lake.
Materials and Methods
Asa dam is located about 5 km South of Ilorin, across River Asa in Ilorin, the capital of Kwara State (08° 26 N, 04° 29' E). The dam was constructed primarily for domestic water supply. The lake, with an area of 302 hectare, has four tributaries river Asa being the major one (Fig. 1). The sampling sites (stations 1 to 3) extended from the dam to Afon, near the river source, where the maximum mean depth at high water levels was 12 m. Close to the dam maximum mean depth was 14 m.
Bimonthly collections of specimens of S. schall for 24 months (March 1991 to February 1993) were carried out at the sampling stations with the services of the local fishermen who were also involved in the keeping of catch records. Gill nets of different mesh sizes including 5.08, 6.35, 7.62, 8.89 and 10.16 cm in a fleet were used to sample randomly the surface, shore and bottom habitats.
Fleet of nets for the shore and surface were in ply 2, while the bottom nets were in ply 4 in order to resist the effect of the bottom pressure which could fold up the net. Each net was 125 m long and it was set at six randomly selected places (i.e. two in each habitat) of the sampling stations making a total of 18 sets of net in all the three stations along the lake. Nets were set at about 6 pm and drawn the following day at about 7 am.
Specimens of S. schall were separated and numbers caught from the different habitats were recorded separately. Each specimen was allotted a serial number after which they were brought to the laboratory in three different ice containers for measuring and weighing. Standard length (cm) and body weight (g) of each specimen were determined using a graduated measuring board in centimetres and a top loading Metler balance in grams respectively. Length and weight measurement were taken to the nearest 0.1. The percentage catches from each habitat was computed and compared statistically using analysis of variance. Also catches from the habitats during the period of wet (May to October) and dry (November to April) seasons were compared statistically. The student t-test analysis was also used for statistical comparison of the catches between the habitats.
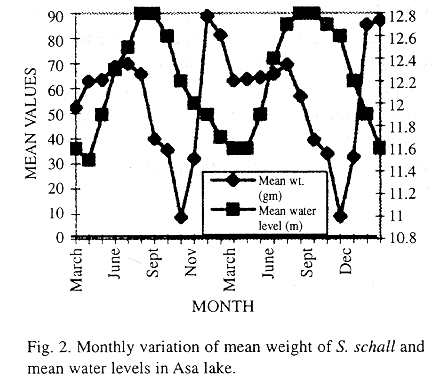
Mean monthly rainfall from March, 1991 to February, 1993 was obtained from Ilorin international airport and mean monthly water levels were recorded from the water level board installed at the dam. The graph of the monthly variation of the mean weight of fish and mean water levels is also presented.
Results
Synodontis schall was caught throughout the year, but highest catches were recorded in January and February, while total catch decreased from September to November when the lake became flooded as a result of increased water levels ( monthly mean range = 12.20 to 12.80 cm) due to the rains that commenced in April (Table 1). Catches within the habitats during the wet and dry seasons were not significantly different (P > 0.05). Similarly catches between the habitats throughout the sampling period were not significantly different (P>0.05).
Monthly variation of mean weight and mean standard length of S. schall and monthly mean values of rainfall and water
levels of Asa lake (March 1991 to February 1993)
| | | (cm) | (cm) | (cm) | (m) | |
| 1991 | March | 35 | | | | |
| April | 38 | | | | | |
| May | 36 | | | | | |
| June | 36 | | | | | |
| July | 33 | | | | | |
| Aug | 37 | | | | | |
| Sept | 26 | | | | | |
| Oct | 21 | | | | | |
| Nov | 22 | | | | | |
| Dec | 32 | | | | | |
| 1992 | Jan | 41 | | | | |
| Feb | 48 | | | | | |
| March | 36 | | | | | |
| April | 39 | | | | | |
| May | 37 | | | | | |
| June | 35 | | | | | |
| July | 33 | | | | | |
| Aug | 37 | | | | | |
| Sept | 27 | | | | | |
| Oct | 22 | | | | | |
| Nov | 23 | | | | | |
| Dec | 32 | | | | | |
| 1993 | Jan | 40 | | | | |
| Feb | 47 | | | | | |
Monthly variation of mean body weight and mean standard length is also shown in Table 1, while monthly variation of body weight and water levels is illustrated in Fig. 2 Lower values of mean standard length and mean body weight were also recorded in October/November.
Monthly variation of the percentage catch within the three habitat is shown in Table 2. They were not caught from the bottom habitat in October/November. All the catches during this month were mainly from the shore and surface habitats hence, the months of October and November recorded highest catches along the shoreline (Table 2). They were caught along the surface throughout the sampling period except in January when the catches were relatively low, and high catches were then recorded at the bottom. This corresponded with the period of dry season when the water level had dropped. The fry and the fingerlings sizes were excluded from the gill net catches.
Monthly variation of total and percentage composition (by mumber) of S. Schall in the surface and bottom
habitats of Asa lake (March 1991 to February 1993)
| Month | catch | shore catch | bottom catch | surface | shore | Bottom | |
| 1991 | March | 11 | 5 | 19 | 31.4 | 14.3 | 54.3 |
| April | 19 | 5 | 14 | 50 | 13.2 | 36.8 | |
| May | 7 | 15 | 14 | 19.4 | 41.7 | 38.9 | |
| June | 10 | 20 | 6 | 27.8 | 55.6 | 16.7 | |
| July | 8 | 15 | 10 | 24.2 | 45.5 | 30.3 | |
| Aug | 13 | 14 | 10 | 35.1 | 37.8 | 27 | |
| Sept | 10 | 1 | 15 | 38.5 | 3.8 | 57.7 | |
| Oct | 7 | 13 | 1 | 33.3 | 61.9 | 4.8 | |
| Nov | 6 | 16 | 0 | 27.3 | 72.7 | 0 | |
| Dec | 12 | 14 | 6 | 37.5 | 43.8 | 18.8 | |
| 1992 | Jan | 3 | 12 | 26 | 7.3 | 29.3 | 63.4 |
| Feb | 9 | 14 | 25 | 18.8 | 29.2 | 52.1 | |
| March | 11 | 7 | 18 | 30.6 | 19.4 | 50 | |
| April | 11 | 6 | 15 | 46.2 | 15.4 | 38.5 | |
| May | 8 | 15 | 14 | 21.6 | 40.5 | 37.83 | |
| June | 10 | 20 | 5 | 28.6 | 57.1 | 14.3 | |
| July | 8 | 15 | 10 | 24.2 | 45.5 | 30.3 | |
| Aug | 13 | 14 | 10 | 35.1 | 37.8 | 27 | |
| Sept | 10 | 2 | 15 | 37 | 7.4 | 55.6 | |
| Oct | 8 | 12 | 2 | 36.4 | 54.5 | 9.1 | |
| Nov | 6 | 15 | 2 | 26.1 | 65.2 | 8.7 | |
| Dec | 12 | 14 | 6 | 37.5 | 43.8 | 18.8 | |
| 1993 | Jan | 4 | 11 | 25 | 10 | 27.5 | 62.5 |
| Feb | 8 | 15 | 24 | 17 | 31.91 | 51.1 | |
| Total | 231 | 290 | 292 | 28.40% | 35.70% | 36.00% | |
Discussion
The presence of S. schall within the three ecological niches was due to its diverse feeding habits. S. schall feeds on a variety of food items including vegetable materials, insects, molluscs, detritus, fish scales and plankton (Motwani;1970, Fagade;1983, Olatunde;1989, Araoye 1997). The flooded littoral zone of the lake was characterised with insect multiplication while the bottom layers were rich in invertebrates particularly molluscs (Araoye 1997). Also a high percentage frequency of occurrence for plants and detritus were recorded in the gut of S. schall during the wet season and these items became more dispersed along the surface water column at this period due to flood and overturn (Araoye 1997).
The ability of S. schall to explore the surface, shore and bottom habitats in search of food items accounted for the insignificant variation of distribution within these niches irrespective of season. Unlike other fish species, the presence of a large physostomous swim bladder, the bony shield of the head and high fat deposition avails this fish the opportunity to explore the bottom habitat despite higher pressure (Willoughby 1976). The low abundance of this fish at the bottom in October/November, when the lake became flooded is attributed to the spawning activities of the fish around this season (Araoye 1997). Over 50% of the adult population were in gonad stages 1V (mature) to V11 (spent) as from September to November (Araoye 1997). The flooded littoral zone at this season provided an expanded ecological niche for shelter to the early life stages after fertilization (Araoye 1997). Also flooding appeared to be a critical exogenous parameter because food (detritus) and other conditions including water current, pH and temperature changes essential for the survival of this species came with flooding to sustain the new recruits into the environment (Ezenwaji 1992).
The paucity of relatively large specimens in the catch at this season was also attributed to restricted movement due to the spawning activities, therefore these sizes became less vulnerable to the gill nets. Berst (1961) reported that the fishing success of the passive nets depends on fish movement and their efficiency and selectivity may be affected by abrupt changes from shift in barometer pressure, wind-driven currents, water level fluctuation, turbidity, and transmitted light. The relative abundance of S. schall irrespective of season can also be due to the success of the fish within the environment due to low predation. Araoye (1997) reported the absence of highly piscivorous fishes such as Lates niloticus, Hydrocynus forskahlii and Gymnarcus niloticus in the lake due to its relatively small size compared with Chad and Kainji lakes. Seasonal abundance of fish species was also reported to be influenced by a combination of physico-chemical properties and the presence of food items (Fagade and Olaniyan 1974).
The inverse relationship between the water level and fish weight may be related with increase in fish concentration due to the draw down of water at dry season. Ita (1978) using experimental gill net data in a bigger lake also observed an inverse relationship between water level and catch rate, explaining the probability of higher concentration of fish at low water levels.
Although this variation may not be significant as reported for S. schall in this report, however the relationship could be significant with commercial catches (Bazigos;1972, Biswas 1973). The report of Bishai and Abu Gideiri (1967) which is at variance with this observation, although not explained by the authors was probably due to the different fishing methods usually embarked upon by the commercial fisheries. The exclusion of fry and fingerlings from the catches in this experiment was due to their non economic sizes which was below 5.08 cm net meshes. The fry and fingerlings of S. schall were usually restricted to the flooded littoral zone of the lake where they feed mainly on zooplankton and insect larvae (Araoye 1997).
This research has shown that a substantial quantity of S. schall can be caught with gill nets set in any of the habitats at any season of the year. Feeding and reproductive phenomena were the main factors responsible for the spatio-temporal distribution of S. schall in Asa lake.
Acknowledgements
I am grateful to C.Y. Jeje and S.O. Fagade, both of the Department of Zoology, University of Ibadan, for their interest and contribution in this work. I also appreciate the co-operation of the local fishermen during field research.
References
Araoye, P.A. 1997. Bio-Ecology of a Mochokid, Synodontis schall (Bloch & Schneider 1801) in Asa dam Ilorin, Nigeria. 201 p. [ Links ]
Bazigos, G.P. 1972. The yield pattern of Kainji lake, Nigeria. FAO, FAO\UNDP\SF\NIR. Rome (No.24). [ Links ]
Berra, T. 1981. An atlas of distribution of the freshwater fish families of the world. University of Nebraska, Lincoln, Nebraska: 191 p. [ Links ]
Berst, A.H . 1961. Selectivity and efficiency of experimental gill nets in South Bay and Georgian Bay of lake Huron. Trans. Am. Fish. Soc. 90: 413- 418. [ Links ]
Bishai, H.M. & Abu Gideiri, Y.B. 1967. Studies on the biology of the genus Synodontis at Khartoum III - Classification and distribution. Rev. zool. Bot. Afr. 2: 62-69. [ Links ]
Bishai, H.M & Abu Gideiri, Y.B. 1968. Studies on the genus Synodontis at Khartoum III. - Reproduction. Hydrobiologia 31: 193-202. [ Links ]
Biswas, S. 1973. Limnological observations during early formation of Volta lake in Ghana. Geophys. Mongor. 17: 121-128. [ Links ]
Egborge, A.B. 1977. The hydrology and plankton of Asejire lake. Ph.D. Thesis, University of Ibadan, Nigeria. 278 p. [ Links ]
Ezenwaji, H.G.M. 1992. The reproductive biology of four African cat fishes (Osteichthyes: Clariidae) in Anambra River Basin, Nigeria. Hydrobiologia 242: 154-164. [ Links ]
Fagade, S.O. 1983. Food and feeding habits of the fishes of Lower River Benue, Nigeria. Bull. de IFAN. Jan. I. T. 45 Ser. A. No. 3-4. 316-314. [ Links ]
Fagade, S.O. & Olaniyan, C.O. 1974. Seasonal distribution of the fish fauna of the Lagos lagoon. Bull. de IFAN . Jan. I. T. Ser. A. 45-67. [ Links ]
Ita, E.O. 1978. An analysis of fish distribution on Kainji lake, Nigeria. Hydrobiologia 58: 233-244. [ Links ]
McConnell, R.H. 1965. Field identification of fresh water fishes likely to occur in the area above the Kainji dam on the River Niger. 43-64 p. In Ed. E. White The first scientific report of the Kainji Biological Research Team. [ Links ]
Motwani, M.P. 1970. Fisheries investigation on the Niger and Benue rivers in the Northern region and development of a programme of riverine fisheries management training. UNDP\FAO Tech. Rep. T.A. 2771. [ Links ]
Nawar, G. 1958. Investigation of the breeding season of some species of Nile fishes. 5th Ann. Rep. Sudan notes Rec. 20-21. [ Links ]
Nawar, G. 1959. The fecundity of the Nile cat fish Synodontis schall (Bloch & Schneider, 1801). Sudan notes Rec. 40: 139-141. [ Links ]
Olatunde, A.A. 1989. Some aspects of the biology of Synodontis schall (Bloch & Schneider, 1801) in Zaria. Nigerian J. Aquat. Sci. 4: 49-54 [ Links ]
Oni, S.K., Olaniyan, J.Y. & Adegboye, J.D. 1983. Comparative physiology of three ecologically distinct fresh water fishes, Alestes nurse Ruppell, Synodontis schall (Bloch & Schneider) and Tilapia zillii Gervais. J. Fish. Biol. 22: 105-109. [ Links ]
Pekkola, W. 1919. Notes on the habitats, breeding and food of some white Nile fish. Sudan notes Rec. 2: 112-121. [ Links ]
Reed, W. Burchad, J. Hopson, A.J., Jennes, J. and Yaro, I. 1967. Fish and fisheries of Northern Nigeria. Publ. M.A.N.R. 22 p. [ Links ]
Sandon, H. & El-Tayed. 1953. The food of some common Nile fishes. Sudan notes Rec. 34: 219-221. [ Links ]
Willoughby, N.G. 1974. The ecology of the genus Synodontis (Pisces: Siluroidei) in lake Kainji, Nigeria, Nigeria. Ph.D. Thesis, University of Southampton. 288 p. [ Links ]
Willoughby, N.G. 1976. The buoyancy and orientation of the upside-down catfishes of the genus Synodontis (Pisces: Siluroidei) J. Zool. Lond. 180: 291-314. [ Links ]
1 Department of Zoology, University of Ibadan, Ibadan, Nigeria. Current address:
Lower Niger River Basin & Rural Development Authority, P.O. Box 5565,
Ilorin, Nigeria.












 uBio
uBio