Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.47 n.1-2 San José Jun. 1999
compartmentalization of photosynthetic pigments
Abstract
Photosynthetic pigments are localized in protein complexes of chloroplast membranes and their role in photosynthesis has long been established but their efficiency has not been measured in many species. The photosynthetic efficiency of four rhizophoracean mangroves, Rhizophora apiculata, R. mucronata, Bruguiera cylindrica and Ceriops decandra was studied in randomly collected propagules from Pichavaram mangrove forest (southeast coast of India) by estimating the concentration of photosynthetic pigments in protein complexes of the thylakoid membrane. Reaction centre chlorophyll (RC-chl) was maximum in B. cylindrica and minimum in R. mucronata. Of the total amount of chlorophylls, RC-chl constitutes about 50%. The light harvesting complex chlorophyll (LHC-chl) was highest in C. decandra and lowest in R. mucronata. Net photosynthesis was found to be higher in B. cylindrica and lower in R. mucronata with the respective CO2 fixation of 20.52 and 10.83 m mol m-2 s-1. A positive correlation was obtained between RC-chl and net photosynthesis. The stomatal conductance to CO2 influx was also found to be high and low in B. cylindrica and R. mucronata respectively. We refer the chlorophylls present in the reaction centre and light harvesting complex as " membrane bound chlorophyll" and propose to use this as an index for measuring the productivity of mangrove species.
Key words
Chlorophylls, mangroves, photosynthesis, rhizophoraceae.
Mangroves are halophytic plants growing along the tropical and subtropical coastline of the world. These plants grow under extreme environmental conditions such as high salinity, temperature and radiation (Ball and Critchley 1982, Bjorkmann et al. 1988, Moorthy 1996). As a result, the mangrove plants exhibit poor photosynthesis and stunted growth. Factors affecting the photosynthesis of mangroves has been little understood (Ball and Farquhar 1984, Clough 1985, Ball et al. 1988, Bjorkmann et al 1988, Kathiresan and Moorthy 1993, 1994) especially dependence of photosynthesis on the photosynthetic pigments. Arnon (1949) has categorized chlorophylls as chlorophyll -a andb. Kathiresan and Kannan (1985) have postulated that photosynthesis of mangroves is dependent on chlorophyll a/b ratio. The photosynthetic pigments are also known to be present in free forms or embedded in the protein complexes of chloroplast membranes (Thornber 1975). Krivosheeva et al. (1991) quantified the bound form of chlorophylls viz., reaction centre (RC) and light harvesting complex (LHC) chlorophylls in pine species, based on their localization. Further, they have also compared the photosynthetic efficiency of the plants with the content of RC - chlorophylls (Krivosheeva et al. 1991). So far, several studies have been made on the relationship between chlorophylls -a, b and photosynthetic activity. Yet, studies relating the presence of chlorophylls in protein complexes of chloroplast and net photosynthesis have not been made on mangroves. Hence, this paper analyses the relationship between compartmentalization of chlorophylls and their role in photosynthesis of rhizophoracean mangroves.
Propagules of Rhizophora apiculata Blume, R. mucronata Lamk., Bruguiera cylindrica (L.) Bl. and Ceriops decandra (Griff.) Ding Hou., belonging to the family rhizophoraceae were randomly collected from the Pichavaram mangrove forest (11- 27'N; 79- 47'E), located in southeast coast of India. Healthy propagules were planted in poly-bags containing estuarine soil and allowed to grow in an open environment (temp. 28 + 2- C; light intensity 1800 moles µm-2 s-1) for 90 days. Plants were irrigated with estuarine water having a salinity of 15 g.l-1 for Rhizophora sp. (Moorthy, 1996) and 8 g.l-1 for Bruguiera and Ceriops sp. (Naidoo 1990). After 90 days, contents of photosynthetic pigments were extracted from leaves with pre-chilled 80% acetone (Arnon 1949). Chlorophyll content in the light harvesting complex (LHC-chl) was estimated from the content of chlorophyll -b, since it presents only in the LHC. The content of chlorophylls present in the LHC of photosystem - II and the content of chlorophyll-a, are in the ratio of 1:1 (Nordenkampf and Lechner 1988). Based on these, content of chlorophyll-a in the reaction centres of the photosystem-I and photosystem-II, was estimated. Net photosynthesis and stomatal conductance were determined by using Li-Cor 6200 portable photosynthesis system. All the analyses were subjected to statistical treatments and the expressed values are average of five replicates.
Photosynthetic capacity can be estimated first to an approximation by measuring the chlorophylls (Greenberg et al. 1992). Hence, photosynthetic pigments present in the protein complexes of reaction centre (RC) and light harvesting complex (LHC) of chloroplast were quantified and the results are presented in Table 1. Of the four mangrove species studied, reaction centre chlorophyll (RC-chl) was the maximum in Bruguiera cylindrica and minimum R. mucronata. The ratio between the levels of the RC-chl and the total chlorophylls was 49.2, 52.3, 48.7 and 48.2% respectively in R. apiculata, R. mucronata, B. cylindrica and C. decandra. It is interesting to note that R. mucronata recorded the lowest RC-chl with the highest ratio of RC-chl/total chlorophylls.
Levels of different types of chlorophylls in four species of mangroves
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
The content of RC-chl was found to have a high positive correlation with the photosynthetic efficiency of mangrove species. The correlation coefficient between the RC-chl and assimilation rate was 0.994, 0.779, 0.967, 0.988 with the regression equations of assimilation rate (X) = 3.33 + 17.36 x RC-chl (Y), x = 16.5 + 6.80 x Y, X = 21.19 - 21.59 x Y, X = 18.67 - 12.94 x Y for R. mucronata, C. decandra, R. apiculata and B. cylindrica respectively. Similar reports have been made earlier in mangrove species, such as R. apiculata, R. mucronata and Avicennia marina (Kathiresan and Moorthy 1993, 1994, 1994a) and pine species (Krivosheeva et al. 1991). Higher content of chlorophyll in reaction centre might enhance the light - induced photosynthetic activity of the chloroplast, thereby high energy transfer (Moorthy and Kathiresan 1993) and energy production could be assumed.
Chlorophylls present in light harvesting complex (LHC-chl) varied from 0.3160 to 0.1884 mg g-1 with the maximum content in C. decandra and minimum in R. mucronata. High content of LHC-chl reflects the better organisation of light harvesting complex in the thylakoid membrane as these chlorophylls act as a structural component (Dahlin 1988) which results in enhanced light trapping efficiency (Lam et al. 1983). Eventhough, there was a significant variations in the content of LHC-chl, the ratio between LHC-chl and total chlorophyll was insignificant among the mangroves species. In all the species, nearly 25% of the total chlorophylls were found to be localised in light harvesting complex. Rhizophora apiculata, R. mucronata, B. cylindrica and C. decandra were determined to contain 25.6, 24.2, 25.6 and 25.6% of LHC-chl respectively.
Reaction centre and light harvesting complex are the two important structural proteins of the thylakoid membranes. Pigments present in these complexes are effectively used by the plants for photosynthetic light harvesting (Goodchild et al. 1972) and energy transfer (Moorthy and Kathiresan 1993). Hence, we propose that, chlorophylls present in these complexes may be referred as "protein - bound chlorophylls". Of the four rhizophorcean mangroves studied, the level of the protein-bound chlorophyll was the maximum in R. mucronata (76.5%) and the minimum in C. decandra (73.8%). The present results reveal that nearly 75% of the total chlorophylls are compartmented either in reaction centre or light harvesting complex and the remaining 25% are present as free pigments. The content of bound chlorophyll was found to have a high correlation with photosynthetic efficiency of mangrove species. The correlation between the protein-bound chlorophylls and assimilation rate was 0.999, 0.977, 0.952, and 0.958 with the regression equations of assimilation rate (X) = 2.546 + 13.16 x bound chlorophyll(Y), 19.23 + 1.30 x Y, 7.95 + 3.6 x Y, 17.64 - 7.52 x Y in R. mucronata, C. decandra, R. apiculata and B. cylindrica respectively.
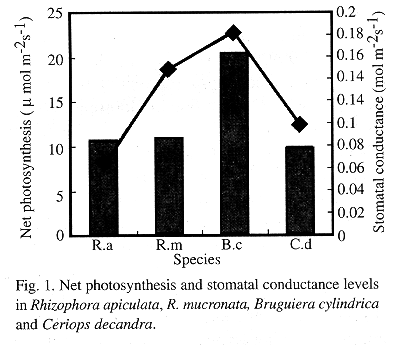
Chlorophyll a/b is an index for determining the photosynthetic efficiency of the mangrove plant system (Kathiresan and Kannan 1985). But, in this study, we claim that ratio between the bound and free forms of chlorophylls can be used as an index for determining the photosynthetic efficiency of the mangrove species. Bound to free chlorophyll ratio ranged from 3.14 to 2.82 with an insignificant variation. High ratio was recorded in R. mucronata and low in C. decandra. This high ratio of bound to free Chl could also be due to presence of more chlorophylls in PS-I, PS-II and LHC complexes. Statistical analysis (correlation co-efficient) for each species revealed that there was in general, a significant variation. In R. apiculata, no correlation was found between photosynthetic productivity and pigments. However, in R. mucronata and B. cylindrica there was a significant positive correlation between net photosynthesis and bound chlorophylls (0.99) and bound to free ratio in R. mucronata (0.98). Kathiresan and Kannan (1985) found a significant productivity and chlorophyll a/b ratio. This prompted us to measure the net CO2 fixation of the four species. B. cylindrica and R. mucronata had net CO2 fixation of 20.52 and 10.83 µmol m-2 s-1; whereas R. apiculata and C. decandra have recorded 10.62 and 9.625 µmol m-2 s-1 (Fig.1) . Stomatal conductance to CO2 was also maximum in B. cylindrica and R. mucronata while R. apiculata and C. decandra showed the minimum among the four species (Fig.1). Bruguiera cylindrica had higher stomatal conductance where the net CO2 fixation was also high. It is concluded from the present study that the chlorophylls present in reaction centre and light harvesting complex could be referred as "membrane-bound chlorophyll"; and also could be used as an index to measure the photosynthetic productivity of mangrove species.
Acknowledgements
The authors are thankful to the Director of this centre, authorities of Annamalai University for providing facilities and to the Council of Scientific and Industrial Research, New Delhi for financial assistance.
References
Arnon, D.I. 1949. Copper enzymes in isolated chloroplasts: polyphenol oxidase in Beta vulgaris. Plant Physiol. 24: 1-15. [ Links ]
Ball, M.C. & C. Critchley. 1982. Photosynthetic responses to irradiance by the grey mangrove Avicennia marina, grown under different regions. Plant Physiol. 70 : 1101-1106. [ Links ]
Ball, M.C..& C.D. Farquhar. 1984. Photosynthetic and stomatal responses of two mangrove species Aegiceras corniculatum and Avicennia marina to long term salinity and humidity conditions. Aust. J. Plant Physiol. 74: 1-6. [ Links ]
Ball, M.C., I.R. Cowan & C.D. Farquhar. 1988. Maintenance of leaf temperature and the optimization of carbon gain in relation to water loss in a tropical mangrove forest. Aust. J. Plant Physiol. 15: 263-276. [ Links ]
Bhatta, R.K. & N.C. Sinha. 1990. Photosynthetic pigments - a prerequisite of grass productivity. Photosynthetica 24: 147-150. [ Links ]
Bjorkmann, I., B. Demming & J.T. Andrews. 1988. Mangrove photosynthesis: Response to high irradiance stress. Aust. J. Plant Physiol. 15 : 43-61. [ Links ]
Clough, B.F. 1985. Effect of nutrient supply on photosynthesis in mangroves. In: The mangroves. Proc. Natl.symp.Biol. Util. Cons. Mangroves. Shivaji University, Kolhapur, India. p 80-88. [ Links ]
Dahlin, C. 1988. Correlation between pigment contribution and apoproterm of the light harvesting complex II (LHC II) in wheat (Triticum aestivum). Physiol. Plant. 74 : 342-348. [ Links ]
Goodchild, O.J., O. Bjorkmann & N.A. Pyliotis. 1972. Chloroplast ultrastructure, leaf anatomy and content of chlorophyll and soluble protein in rainforest species. Carnegie Inst. 71 : 102-107. [ Links ]
Greenberg, B.M., X.D. Huang & D.G. Dixon. 1992. Application of the aquatic plant Lemna gibba for ecotoxicological assessment. J. Aquatic. Ecosystem Health. 1: 147-155. [ Links ]
Kathiresan, K. & L. Kannan. 1985. Photosynthetic productivity in species of Rhizophora. In: The Mangroves. Proc. Natl. Symp. Biol. Util. Cons. Mangroves Shivaji university, Kolhapur, India. p 262-265. [ Links ]
Kathiresan, K. & P. Moorthy. 1993. Influence of different irradiance on growth and photosynthetic characteristics in seedlings of Rhizophora species. Photosynthetica 29: 143-146. [ Links ]
Kathiresan, K. & P. Moorthy. 1994. Photosynthetic responses of Rhizophora apiculata Blume seedlings to a long-chain aliphatic alcohol. Aquat.Bot. 47: 191-193. [ Links ]
Kathiresan, K. & P. Moorthy. 1994a. Hormone-induced physiological responses of a tropical mangrove species. Bot Mar. 37: 139-141. [ Links ]
Krivosheeva, A., S.A. Shavnin, V.A. Kalinin & P.S. Venedikov. 1991. Effect of industrial pollutants on seasonal changes of chlorophyll content in scotch pine seedlings. Fiziol.Rastenii. 38: 162-168. [ Links ]
Lam, B., B. Baltimore., N. Ortiz., W. Chollars., A. Melis & R. Malkin. 1983. Characterisation of a resolved oxygen evolving photosynthesis preparation from Spirulina thylakoid. Biochem. Biophys. Acta. 724: 201-211. [ Links ]
Moorthy, P. 1996. Effects of ultraviolet-B radiation on mangrove environment: Physiological responses of Rhizophora apicualta Blume. Ph.D thesis, Annamalai University, India. 130p. [ Links ]
Moorthy, P. & K. Kathiresan. 1993. Physiological responses of a mangrove seedling to triacontanol. Biol. Plant. 35: 577-581. [ Links ]
Naidoo, G. 1990. Effects of nitrate, ammonium and salinity on growth of the mangrove Bruguiera gymonorhiza (L.) Lam. Aquat. Bot. 38 : 209-219. [ Links ]
Nordenkampf, H.B. & E. Lechner. 1988. Temperature and light dependent modification of chlorophyll fluorescence kinetics in spruce needle during water stress. Photosynthetic Res. 18: 287. [ Links ]
Thornber, J.P. 1975. Chlorophyll proteins light harvesting and reaction centre components of plants. Ann. Rev. Plant Physiol. 26 : 127-158. [ Links ]
1 Centre of Advanced Study in Marine Biology, Annamalai University, Parangipettai 608 502, Tamil Nadu, India.
*Present Address: Department of Biological Sciences,The National University of Singapore, 119260 Singapore, Fax: 65 7792486; e-mail: scip7035@nus.edu.sg