Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.46 n.3 San José Sep. 1998
Resumen
En un muestreo hecho de febrero a junio en Puntarenas, Costa Rica, cuatro de 20 árboles de Psedolmedia oxphylaría (Moraceae) tenían agallas del insecto Trichochermes magma. Un 7% de las agallas mostraban signos de parasitación o depredación. La mayoría de las agallas estaban en hojas jóvenes, pero no hubo correlación entre tamaño de hoja y número de agallas. La ninfa tiene una estructura en forma de cuerno que podría servirle de defensa.
Key words
Defense, host plant use, morphology, parasitoids.
The superfamily Psylloidea contains around 2000 species of Stenorrhyncha (Homoptera) that are characterized by rear legs adapted for jumping (Earstop 1978). The nymphs feed mainly on phloem, and some species induce the formation of galls in plants (Woodbum and Lewis 1973; Hodkinson 1973; Clark 1962). This group includes potential pests in forests and is particularly rich in species in neotropical forests. In Costa Rica, they feed on several native trees, such as Alaroa, Cedrella, Haematoxylum, Hymenaea, Inga, Lonchocarpus, Pentaclethra and Virola (Noyes and Hanson 1996).
The following note includes observations on the distribution, size and behavior of the nymphs of the gall-former Trichochermes magna on Psedolmedia oxyphylaria.
The galls were collected from February to June in the Monteverde Reserve Forest, Puntarenas, Costa Rica. All the new leaves with galls and a similar number of old leaves were collected. The number, size and distribution of the galls on the leaf were measured. The galls were dissected traversely so that the animal was observed dorsally. Voucher specimens were deposited in the Museo de Insectos, University of Costa Rica.
Only four of 20 host plant trees Psedolmedia oxphylaría (Moraceae) had developing galls; Trichochermes magma forms a rounded, hemispherical gall with thorn-like prolongation. Each gall had a single chamber that contained only one nymph. Developing galls were present on new leaves only. When the adult was ready to emerge, the gall expanded outward and opened like a flower. Opened galls were more abundant on old leaves. Of 257 galls examined 39. 7% showed perforations of various sizes that suggested mortality caused by predators and parasitoids. Three parasitoids, Psyllaephagus ufens (Encyrtidae) (Noyes and Hanson 1996) and two species of the family Eulophidae (subfamily Tetrasichinae), were reared
There were an average of 33. 7 galls per leaf (n=54), and of those 65% were present on "new leaves" (38. 2±31. 6 galls per leaf, n =35) and 35% on "old" ones (18. 5±13. 4, n= 19, P<0. 01, t= 2. 77). The average diameter of galls on "young" leaves was 2. 2±1. 9 mm (n= 524) and on mature leaves 4. 1±0. 7 mm (n= 222) (P<O. Ol, t=5. 69). There was no correlation between size of the leaf and number of galls on it (P< 0. 01, r= 0. 0576). Of the developing galls 7% contained eggs, 13. 8% first instar nymphs, 20. 8% second instar, 25% third ínstar, 20. 2 fourth and 13. 2% fifth instar nymph (n= 144).
The nymph, is robust and dorsoventrally flattened with dorsal sclerites of the thorax fused. The labium and the antennae are relatively short, and the legs are not functional. A horn projects from the head líke a rhinoceros horn (Fig l. ). In the fifth instar the thorax and head are yellow and the abdomen is intense light green.
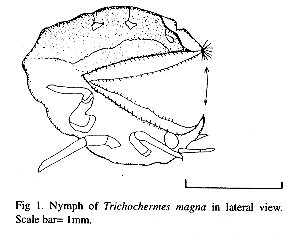
Typically the nymph rested in the gall with its body curved and the horn oriented toward the apex of the gall. When the larva's head was experimentally stimulated with an insect pin, the horn was tossed quickly in the direction of the stimulus toward the opening of the gall (n=31, P<0. 01, x2 test). Stimulation of the abdomen provoked the animal to curve quickly and swing it several times like a scorpion (n=35, P<0. 01, x2 test). When the nymph was stimulated in the middle part (near the junction of the abdomen and the thorax), it responded by repeatedly flexing and closing both parts of the body on the pin (n=40, P<0. 01, x2 test). When the animal was stimulated repeatedly it oriented the ventral part of the abdomen toward the opening of the gall. This behavior resembles that of Trioza ru1sellae (Triozidae) on Brosimun sp. in which the nymph covers the opening of the gall with a sclerotízed plate (P. Hanson, pers. comm. ). However, in T. magna there is no plate rather the intense green colored abdomen is exposed.
Apparently this species is capable of developing a full nynfal cycle when a leaf is young. The high density of galls found on some of the leaves produced distortions in the growth that induce a severe corrugation.
The galls of psylids are through to protect them from extreme climatic conditions, particularly drying, and from parasitoids and predators (Hodkinson 1973). Many galls of T. magna however showed damage apparently occasioned by predators and/or parasitoids. Probably, the evolution of the horn and associated behaviors was evolved to aid in active defense during the nymphal stage against such enemies.
Acknowledgements
D. Hollis identified the psylids, P. Hanson the parasitoids and J. Gómez the plants. W. Eberhard and P. Hanson made suggestions and comments on the manuscript. This study was financed by the Vicerrectoría de Investigación of the University of Costa Rica.
..
References
Brown R-G. & 1. D. Hodkindon. 1988. Taxonomy and ecology of the jumping plant-lice of Panama. Entomograph 9. 304 p. [ Links ]
Clark, L. R. 1962. The general biology of Cardiaspina albitextura (Psyllidae) and its abundance in relation to weather and parasitism. Aust. J . Zool. 10: 537-586. [ Links ]
Eastop, V. F. 1973. Deductions from the present day host plants of aphids and relates insects, p. 157-178. In H. F van Emden,. Insect/Plant Relationships. Royal Entomological Society of London. Symposium 6. Edward Arnold, London. [ Links ]
Hodkinson I. D. 1974. The Biology of the Psylloidea (Homoptera): a review. Bull. Ent. Res 64: 325-339. [ Links ]
Hodkinson, I. D. , 1984. The biology and ecology of gall-forming Psylloidea, p. 59-78. In T. N. Ananthakrishnan (ed). Biology of Gall Insects. Oxford & IBH New Delhi, India. [ Links ]
Noyes, J. S. & P. Hanson. 1996. Encyrtidae (Hymenoptera: Chalcidoldea) of CostaRica: the genera and species associated with jumping plant-lice (Homoptera: Psylloidea). Bull. Nat. Hist. Mus. Lond (Ent) 65(2): 105-164. [ Links ]
Woodbum, T. L. & E. E. Lewis. 1973. A comparative histological study of the effects of feeding by nymphs of four psyllid specíes on the leaves of eucalyptus. J. Aust. Ent. Soc 12: 134- 138. [ Links ]
1Escuela de Biologia, Universidad de Costa Rica, 2060 San José, Costa Rica, rbriceno@cariari.ucr.ac.cr














