Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.46 n.3 San José Sep. 1998
Abstract
Spatial heterogeneity of a Schinopsis balansae forest ("Quebrachal") near Vera (Santa Fe, Argentina) was studied for any correlation between woody species distribution and environmental factors. This type of forest is the most important community of the Santa Fe Forest Wedge, which is the southernmost portion of the Eastern Chaco. Thirty two 10 x 10 m plots were laid along two transects. Woody vegetation and microzones determined for microrelief, soil moisture and the presence of bromeliads were mapped; 54% of the ground is level, about half of it is muddy or frequently flooded, and little more than 11% is concave almost always flooded. Convex soils are well drained and often covered by populations of spiny bromeliads. Most woody individuals were clumped on high and well drained soils while only Geoffroea decorticans, Prosopis spp. and S. balansae were present on very humid microzones. These results strongly suggest that soil heterogeneity (microrelief and soil moisture), is the most important factor that determines the distribution of woody species.
Key words
Anacardiaceae, Argentina, Chaco, environmental heterogeneity, forests, microrelief, microsites, Schinopsis balansae.
Natural systems have been recognized as abiotically heterogeneous in space and time, and variable in organism abundance (Sousa 1984). Since the spatial heterogeneity of the environment affects many aspects of the population biology of plants, such as population dynamics, seed dispersal and plant recruitment (Harper 1977, Grubb 1977), it is therefore a major factor regulating the distribution of individuals and species (Urban et al. 1987). The characteristics of the habitat determine which individuals from those that arrive at a place, actually occupy that particular place (Bazzaz 1991). In a community, the floristic composition is determined by the differential response of species to the present or past biotic and abiotic conditions (Gleason 1926, Whittaker 1967).
The Chaco region (15o to 35oS) is a very large sedimentary plain that covers about 1 000 000 km2 of eastern Bolivia, western Paraguay, northern Argentina and a small part of southeastern Brasil (Prado 1993). The whole region is not homogeneous, there is a humid Chaco at the east and a dry one at the west (Ragonese and Castiglioni 1970). During the Holocene differential movements of bedrock blocks of the Eastern Chaco, produced contrasting landscapes that were further modified by fluvial activity (Popolizio et al. 1980). At the local level topography produces strong ecological differences, that results in the presence of different plant formations (Morello and Adámoli 1974, Lewis 1991).
The southernmost segment of the Eastern Chaco is the Santa Fe Forest Wedge ("Cuña boscosa"), which is located between the Submeridional Lowlands (Spartina argentinensis Parodi grasslands with almost no trees) at the west and the Paraná river at the east from parallel 30o30'S to 28oS. The climate is temperate-warm humid with a mean annual precipitation slightly above 1 000 mm, which occurs mainly in summer, and with a strong winter drought of variable length (Burgos 1970). The vegetation is a mosaic of forests, savanna grasslands and hygrophilous communities ordered along moisture and salinity gradients correlated with topographic elevation (Lewis and Pire 1981). The Schinopsis balansae Engl. forest ("Quebrachal") appears on halo-hydromorphic soils (Espino et al. 1983), in the middle of the elevation gradient between Prosopis nigra (Gris.) Hier. var. ragonesei Burk. forest ("Algarrobal") at the lower places and the mixed dense forest ("Bosque chaqueño") at the higher places (Lewis and Pire 1981, Lewis 1991), and it is the most important community of the region, quite different from other forests where Schinopsis species may be present (Lewis and Pire 1981, Prado 1993). Several aspects of this forest have been studied by Ragonese and Covas (1940) and Lewis and Pire (1981). Lewis (1991) suggested that the clumped distribution of tree individuals in this forest is the result of the spatial heterogeneity and that microzones are colonized differentially by woody species. However, a detailed analysis of its spatial heterogeneity and the development of its pattern have not been carried on so far. The object of this paper is to analyse the spatial heterogeneity of one stand of a S. balansae forest near Vera (Santa Fe, Argentina) and to establish whether there is any correlation between the distribution of the woody species and environmental factors. The underlying hypothesis is that the pattern of the woody layers is correlated to some soil characteristics, mainly microrelief and edaphic humidity.
Materials and Methods
The study area: It is located in the experimental center of the Provincial Ministry of Agriculture at Las Gamas, near Vera (29o28'S - 60°28'W), Province of Santa Fe. The forest was heavily lumbered in the first half of this century for tannin industry and subsequently for fuel extraction and charcoal production. This stand, although is partially recovered, is not completely mature or at a steady state, and cattle grazing, mild wood extraction and sporadic fires are the main disturbances.
Data collection and analysis: On a transect 22 contiguous 10 x 10 m plots were laid (Lewis 1991) and another ten plots of the same size were laid on a second discontinuous transect parallel to the first one. On March 1989, all trees taller than 1.6 m and shrubs taller than 0.6 m present in the plots, were counted and mapped and at the same time, soil surface was divided in microzones that were also mapped. Microzones were determined according to microrelief (D: concave, P: level, E: convex; margins of these areas were characterized as: m: stepped n: sloped), soil moisture (1: well drained, 2: dry, 3: muddy, 4: swampy and 5: flooded) and the presence of bromeliads and small mounds in ponds. Relative topographic elevation of 40 points on different microzones along one of the transects, was measured with an optical level. With these data, cartographic diagrams of vegetation and microzones were drawn on a grid of quadrats at a scale of 1: 100.
Microzones were grouped in the following categories: convex with bromeliads (EB), convex without bromeliads (E), level most dry (PS, that includes P1, P2 and P3), level most humid (PH, that includes P4 and P5) and concave (D). Microzones are not continuous over the transect plots, but divided in several sectors each, and each sector can be spread in one, two or more plots.
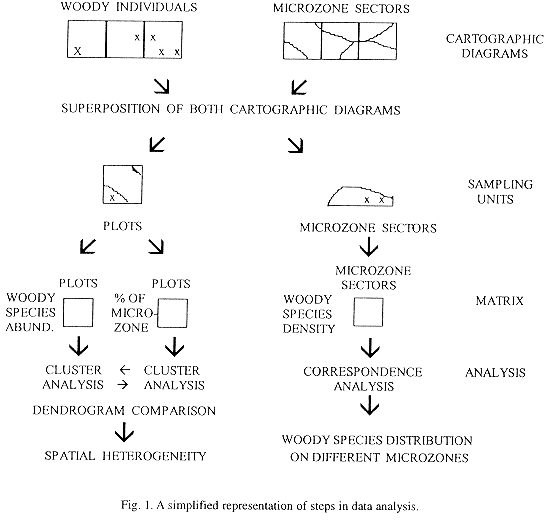
Results were analysed following two different approaches (Fig. 1). First, to analyse the spatial heterogeneity two cluster analyses of plots with complementary data matrix were performed. Plots were classified according to the number of trees taller than 1.6 m and shrubs taller than 0.6 m, with Mulva's (Wildi and Orlóci 1990) Minimal Variance Clustering technique using van der Maarel's coefficient to construct the resemblance matrix. This technique and similarity measure were chosen because they proved to be the most robust technique available for these data (Torres et al. 1995). Plots were also classified according to the surface proportion of different microzones with farthest neighbour technique, using Sörensen coefficient to construct the resemblance matrix (McCune and Mefford 1995). In this case this technique was used because it is space-dilating, so group separation is more evident (Greig-Smith 1983).
To analyse woody species distribution on different microzones an ordination method was performed. Microzone diagrams were compared by superimposing them on the cartographic diagrams of vegetation, and the number of individuals of woody species in each microzone sector was calculated, and results were later corroborated in the field. The area of the microzone sectors was estimated with an area-meter. The area of different sectors of microzones is very variable ranging from 20 to 315 m2, therefore, in order to compare woody species distribution on microzones sectors, density provides a more appropiate measure than the number of individuals. Woody species and microzone sectors were ordered according to the density of individuals in each sector by means of Correspondence Analysis and Decorana from Pc-ord (McCune and Mefford 1995). Default option of Decorana was used. Only those sectors with an area higher than 20 m2 were analysed, depressed sectors (D) and species with less than three individuals were not taken into account to avoid noise and distortion of results.
Results
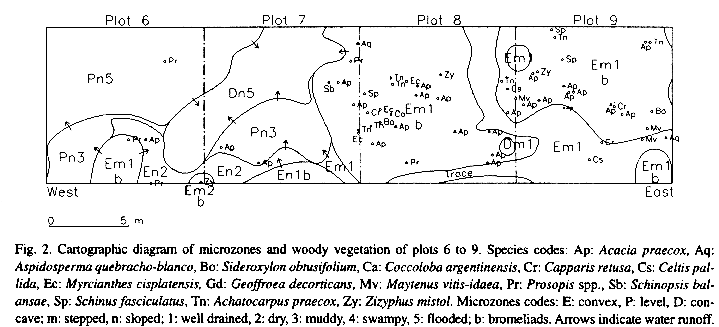
Individuals of woody species are clumped, therefore tree canopy is very dense in some places, but nevertheless discontinuous, so that all along the transects there are gaps that can be rather large (Fig. 2). The large gaps are covered by a dense understory of grasses, while the more shaded parts of the forest are invaded by two spiny bromeliads (Aechmea distichantha Lemaire and Bromelia serra Gris.).
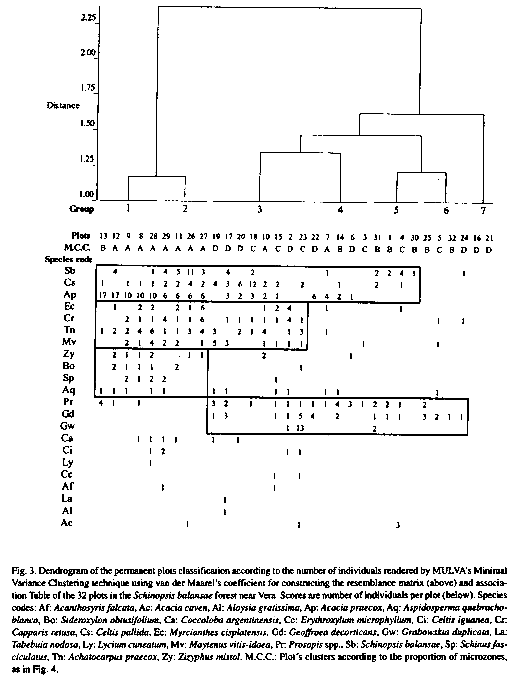
Plots along the transects show differences in their floristic composition and when they are classified, they form two main groups, one with dense woody vegetation and the other with more sparse woody vegetation (Fig. 3). The first group is divided in two subgroups (1 and 2) according to the relative abundance of Acacia praecox Gris. and S. balansae. In the second one, two plots without any individuals at all, are grouped together (7), appart from most other plots which are clustered in four groups (3, 4,5 and 6) floristically quite different from each other as shown in the dendrogram and Table. Zizyphus mistol Gris., Sideroxylon obtusifolium (Roem. et Schult.) Pennington, Schinus fasciculatus (Gris.) Johnst. and Aspidosperma quebracho-blanco Schlecht. are characteristic species of the first main group, in contrast to Geoffroea decorticans (Hook. et Arn.) Burk. and to a lesser degree, Prosopis spp. (P. alba Gris., P. nigra (Gris.) Hieron. and their hybrids), which are characteristic species of the second one (Fig. 3).
The distribution of microzones defined by the microtopography shows a gradual transition in some places, such as plots 6 and 7, while in others there is an abrupt change as in the most part of the boundary between plots 7 and 8 (Fig. 2). There are also very small ponds in the higher microzones like the one near the limit between plot 8 and 9 or larger ones like one in the southern part of plots 10 and 11 (not shown in Fig. 2). The bromeliads occur only in the highest and better drained microzones, but not all higher microzones necessarily have bromeliads. In relatively deep ponds, small hummocks near the edges may be common.
The most humid microzones, or the best drained ones are in concave areas or conversely in the convex parts of the ground, but this does not mean that they are the lowest or highest parts of the ground, but only relatively so. Most of the area, (little more than 54 %) is flat, half of it swampy or frequently flooded, and only little more than 11 % is concave most of which is often flooded. Fig. 2 is a fairly representative sample of the distribution of microzones, although as a whole the analysed area has a lower proportion of well drained soils.
The distribution of microzones among plots is very variable, so when plots are classified according to microzone proportion, they form two main clusters. One of them, groups plots which are mostly with a convex microrelief (A) and the other one, groups plots which have a lower proportion of convex microrelief. The second main cluster is subdivided in a group with plots mainly dry level (B), and another which is also divided in two groups, the first with plots mainly humid level (D) and the second one with plots with a mixture of microzones (C) (Fig. 4).
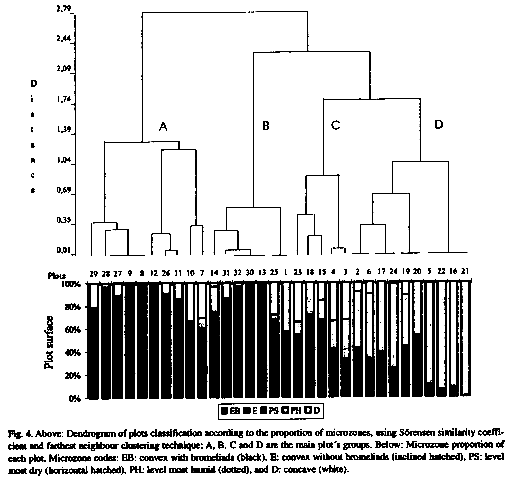
Most individuals of woody species are distributed mainly on well drained convex and level soils. Very few individuals occur on the concave and very wet soils (Fig. 2); while well drained soils account for only 35 % of ground surface, 75 % of the woody species individuals appear on them. If the classifications of plots according to their floristic composition and the proportion of microzones are compared, at first sight appears a rather narrow correspondence between them. For example, plots of groups 1 and 2 which have more individuals of woody species, as a whole belong to plot group A with larger proportion of higher soils (EB and E), while most plots of groups 5 and 6 belong to plot group B and the empty plots (group 7) are in plot group D with large proportion of humid soils (Fig. 3 and 4).
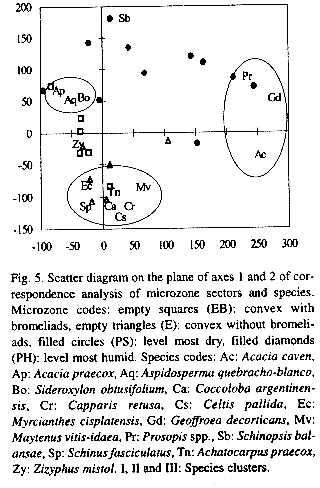
Correspondence analysis of the microzones sectors segregates to the right of the first and to the upper part of the second axes the most humid ones and to the left and the bottom of the same axes, the best drained microzones (Fig. 5). The same analysis of the species, groups them in an open cluster (I) with Prosopis spp., G. decorticans and Acacia caven (Mol.) Mol. on the right of the first axis and all other species to the left in two clusters, one (II) with Acacia praecox, A. quebracho-blanco and S. obtusifolium in the upper side of axis 2, and another (III) with Myrcianthes cisplatensis (Camb.) Berg., Achatocarpus praecox Gris., Coccoloba argentinensis Speg., Celtis pallida Torrey, Capparis retusa Gris., Maytenus vitis-idaea Gris., and S. fasciculatus to the bottom of the same axis, thus leaving Z. mistol between both clusters and S. balansae in the uppermost part of the diagram (Fig. 5). If the same analysis is done with Detrended Correspondence Analysis (Decorana) (Hill and Gauch 1980), the results (not shown here) are similar, but with all species of the dry sites at the left side of axis 1, apart from S. balansae, in a very compact cluster, that cannot be segregated on axis 2.
Discussion
The tree layer of this stand is spatially very heterogeneous (Lewis et al. 1997) and plots are grouped in high resolution clusters. While plots form well defined clusters, floristic groups are not so well defined, and plot clusters are characterized more by the relative abundance of the species, rather than by their floristic composition. If the classification is done with the same method, but using as resemblance matrix the cross product of non center data matrix, results are almost the same. Moreover, the dendrograms were quite similar to that published by Lewis (1991) constructed using only 22 of these plots.
The microzone maps (Fig. 2) show that the soils have irregular microtopography, which in certain places is markedly contrasting. Water accumulates in the concave parts of the soil which are not necessarily the lowest parts. Therefore the wettest soils are in the deepest parts of depressions while convex soils are always well drained. Hummocks in the edges of some ponds seem to be the result of erosion produced by livestock trampling. Bromeliads colonize the relatively higher microzones, but as some of the convex and well drained soils have no bromeliads at all, some other factor, perhaps light intensity, may condition their presence in the relatively high soils (Brokaw 1983, Lee et al. 1989). Also the two species of bromeliads tend to be segregated from each other.
The spatial heterogeneity of this stand can be indicated by tree distribution within the plots, which as shown above, is clumped (Fig. 2). As pointed out by Palmer and Dixon (1990), many plots may not represent a single point on any of the environmental gradients, but rather occur over a segment of certain length. The analysis of plots in this forest shows that there is a correspondence between the plots which have a high number of trees and higher and better drained microzones.
Aggregated distribution of woody individuals have been linked to different factors such as: seedfall patterns (Hubbell 1979), edaphic conditions (Turner and Franz 1985) or canopy structure (Augspurger 1984). In this forest the analysis of the distribution of woody species in different microzones, strongly supports the hypothesis that the clumped distribution of trees is the result of edaphic or topographic heterogeneity as it was previously suggested by Lewis (1991). Also as in many other Chaquenian forests, there are islands of trees interwoven with grass patches, the latter usually found in relatively lower topographic position (Morello and Adámoli 1974). Similar causes have been suggested for the clumped distribution of woody species in Chaco-related and non-related communites of the Pantanal in Mato Grosso (Prado et al. 1992, Adámoli 1995)
The slight differences between both ordination analyses to determine the distribution of woody individuals on microzones, may be the result of an artifact of Decorana (Wartenberg et al. 1987). The analysis shows that most woody species do not grow on often flooded soils, though some individuals of trees and shrubs apparently grow on depressed microzones; however, field inspection showed that most of them were on relatively high ground or little elevations out of scale within these microzones. Nevertheless, distribution of species on different microzones is not uniform, Acacia praecox, M. cisplatensis, C. pallida, C. retusa and most species occur on dry soils and cannot be on lower and very wet soils, while G. decorticans, Prosopis spp. and A. caven grow on wetter soils. S. balansae can grow on more humid soils than the first group, and actually in some places it appears next to species of the second group, but grows on well drained soils as well.
Prosopis spp., G. decorticans and A. caven, which are present mainly in the most humid soils in this forest, are the dominant woody species of the Peristeppic Thornforest which is at the south of the Santa Fe Forest Wedge (Lewis and Collantes 1973), whereas most other species which thrive in well drained soils of this forest are not present at all in the Peristeppic Thornforest. Moreover the Amazonian lineage species (sensu Prado 1991) are present neither in the most humid soils of this forest nor in the Peristeppic Thornforest.
Soil heterogeneity, mainly microrelief and moisture, is the most important factor that determine the distribution of species on the ground. However, how this heterogeneity develops is in need of further research, and several hypotheses can be put forward. The irregularity of the surface may be the result of the physiographic history of the site, pseudokarstic processes and wind deflation as well as hydraulic accumulation processes, but also plant-soil and animal-soil interactions such as trampling and the building activity of ants and other small animals should be considered (Bucher 1980, Popolizio et al. 1980, Lewis 1991).
The above discussion on microsites considered only microzones defined by microrelief and soil characteristics associated with it. However, each one of these microzones are further divided by the aerial environment, fundamentally by the light climate; there are places exposed to full irradiation, and others on full shadow, with all intermediates. Also, it should be taken into account that there are more than one layer of woody species which are not continuous, leaves are of different types and some species are deciduous and others evergreen (Lewis et al. 1997). So far, only a very coarse qualitative characterization of the forest microclimate can be done. Therefore, this aspect is in need of further research in order to have a quantitative characterization of microhabitats. In addition to these static aspects of the spatial heterogeneity of the microsites, there are also dynamic processes at work, such as changes in herbaceous/woody species caused by overgrazing (Morello 1970, Adámoli et al. 1990), gallery forest dynamics resulting from intensive river-bed migration processes (Adámoli et al. 1990, Sennhauser 1991) and other processes as well, that also need further research.
Acknowledgements
Financial aid from the CONICET (Council for Scientific and Technical Research, Argentina) is gratefully acknowledged. The authors are indebted to the Santa Fe Ministry of Agriculture for logistic support and to the staff of Las Gamas experimental Center for their invaluable help. J.L. Vesprini and E. Brnich provided invaluable help during field work and P.S. Torres and J.R. Fernández with the computing work. D.E. Prado and E.A. Franceschi read critically the manuscript and made very useful suggestions.
Resumen
El objeto de este trabajo es analizar la heterogeneidad espacial de un bosque de Schinopsis balansae ("Quebrachal") cerca de Vera (Santa Fe, Argentina) y establecer si hay correlación entre la distribución de las especies leñosas y los factores ambientales. Este tipo de bosque es la comunidad vegetal más importante de la Cuña Boscosa de Santa Fe, que es la porción más austral del Chaco Oriental. En un stand parcialmente recuperado se instalaron 32 parcelas contiguas de 10 x 10 m a lo largo de dos transectas. Se cartografiaron todos los individuos de las distintas especies de leñosas, así como los distintos micrositios caracterizados por el microrelieve, humedad del suelo y presencia de bromeliáceas terrestres. El 54% del suelo es plano, la mitad del cual es barroso o está frecuentemente inundado, y algo más del 11% es cóncavo y está casi siempre inundado. La mayor parte de las leñosas crecen en micrositios bien drenados mientras que en suelos muy húmedos solamente crecen Geoffroea decorticans, Prosopis spp. y S. balansae. Los resultados obtenidos sugieren que la heterogeneidad del suelo, (microrrelieve y humedad) es el factor más importante que determina la distribución de las especies leñosas sobre el terreno.
References
Adámoli, J. 1995. Diagnóstico do Pantanal: (Características ecológicas e problemas ambientais). Programa Nacional do Meio Ambiente, Brasilia. 50 p. [ Links ]
Adámoli, J., E. Sennhauser, J.M. Acero & A. Rescia. 1990. Stress and disturbance: vegetation dynamics in the dry Chaco region of Argentina. J. Biogeogr. 17: 491-500. [ Links ]
Augspurger, C.K. 1984. Seedling survival of tropical tree species: interactions of dispersal distance, light gaps, and pathogens. Ecology 65: 1075-1712. [ Links ]
Bazzaz, F.A. 1991. Habitat selection in plants. Am. Nat. 137 (suppl.): 116-130. [ Links ]
Brokaw, N.V.L. 1983. Groundlayer dominance and apparent inhibition of tree regeneration by Aechmea magdalenae (Bromeliaceae) in a tropical forest. Trop. Ecol. 24: 194-200. [ Links ]
Bucher, E.H. 1980. Ecología de la fauna chaqueña. Una revisión. Ecosur 7: 111-221. [ Links ]
Burgos, J.J. 1970. El clima de la región noreste de la República Argentina. Bol. Soc. Arg. Bot. 11 (supl.): 37-102. [ Links ]
Espino, L.M., M.A. Seveso & M.A. Sabatier. 1983. Mapa de suelos de la Prov. de Santa Fe. Tomo II. MAG Santa Fe-INTA EERA Rafaela, Argentina. 220 p. [ Links ]
Gleason, H.A. 1926. The individualistic concept of the plant association. Bull. Torrey Bot. Club 53: 7-26. [ Links ]
Greig-Smith, P. 1983. Quantitative plant ecology. Studies in Ecology Vol. 9. University of California, Berkeley. 359 p. [ Links ]
Grubb, P.J. 1977. The maintenance of species richness in plant communities: the importance of the regeneration niche. Biol. Rev. 52: 107-145. [ Links ]
Harper, J.L. 1977. Population biology of plants. Academic, London. 492 p. [ Links ]
Hill, M.O. & H.G. Gauch, Jr. 1980. Detrended correspondence analysis: an improved ordination technique. Vegetatio 42: 47-58. [ Links ]
Hubbell, S.P. 1979. Tree dispersion, abundance, and diversity in a tropical dry forest. Science 203: 1299-1309. [ Links ]
Lee, H.S.J., U. Lüttge, E. Medina, J.A.C. Smith, W.J. Cram, M. Díaz, H. Griffiths, M. Popp, C. Schafer, K.H. Stimmel & B. Thonke. 1989. Ecophysiology of xerophytic and halophytic vegetation of a coastal alluvial plain in northern Venezuela. III. Bromelia humilis Jacq., a terrestrial CAM bromeliad. New Phytol. 111: 253-271. [ Links ]
Lewis, J.P. 1991. Three levels of floristical variation in the forests of Chaco, Argentina. J. Veg. Sci. 2: 125-130. [ Links ]
Lewis, J.P. & M.B. Collantes. 1973. El espinal periestépico. Ciencia Investigación 29: 360-377. [ Links ]
Lewis, J.P. & E.F. Pire. 1981. Reseña sobre la vegetación del Chaco santafesino. Serie Fitogeográfica 18. INTA, Buenos Aires. 42 p. [ Links ]
Lewis, J.P., E.F. Pire & I.M. Barberis. 1997. Structure, physiognomy and floristic composition of a Schinopsis balansae (Anacardiaceae) forest in the Southern Chaco, Argentina. Rev. Biol. Trop. 45: 1013-1020. [ Links ]
McCune, B. & M.J. Mefford. 1995. PC- ORD. Multivariate analysis of ecological data, Version 2.0. M.J.M. Software Design, Gleneden Beach, Oregon. 126 p. [ Links ]
Morello, J. 1970. Modelos de relaciones entre pastizales y leñosas colonizadoras en el Chaco Argentino. IDIA 276: 31-52. [ Links ]
Morello, J. & J. Adámoli. 1974. Las grandes unidades de vegetación y ambiente del Chaco argentino. Segunda parte: vegetación y ambiente de la provincia del Chaco. Serie Fitogeográfica 13. I.N.T.A., Buenos Aires. 130 p. [ Links ]
Palmer, M.V. & P.M. Dixon. 1990. Small-scale environmental heterogeneity and the analysis of species distributions along gradients. J.Veg. Sci. 1: 57-65. [ Links ]
Popolizio, E., P.Y. Serra & G.O. Hortt. 1980. Bajos Submeridionales. Grandes unidades taxonómicas de Chaco. Centro de Geociencias Aplicadas. Serie C - Investigación. Resistencia, Chaco, Argentina. 205 p. [ Links ]
Prado, D.E. 1991. A critical evaluation of the floristic links between Chaco and Caatingas vegetation in South America. Ph. D. thesis, University of St. Andrews, St. Andrews, Scotland. 420 p. [ Links ]
Prado, D.E. 1993. What is the Gran Chaco vegetation in South America? I. A review. Contribution to the study of flora and vegetation of the Chaco. V. Candollea 48: 145-172. [ Links ]
Prado, D.E., P.E. Gibbs, A. Pott & V.J. Pott. 1992. The Chaco-Pantanal transition in southern Mato Grosso, Brazil, 451-470. In: Furley, P.A., J. Proctor & J.A. Ratter (eds.). Nature and dynamics of forest-savanna boundaries. Chapman & Hall, London. [ Links ]
Ragonese, A.E.& G. Covas. 1940. La distribución geográfica de los quebrachales en la provincia de Santa Fe. Rev. Arg. Agron. 8: 176-184. [ Links ]
Ragonese, A.E. & J.C. Castiglioni. 1970. La vegetación del parque chaqueño. Bol. Soc. Arg. Bot. 11 (supl.): 133-160. [ Links ]
Sennhauser, E. 1991. The concept of stability in connection with the gallery forests of the Chaco region. Vegetatio 94: 1-13. [ Links ]
Sousa, W.P. 1984. The role of disturbance in natural communities. Annu. Rev. Ecol. Syst. 15: 353-391. [ Links ]
Torres, P.S., I.M. Barberis & J.P. Lewis. 1995. Robustness of numerical methods for vegetation classification. Coenoses 10: 11-16. [ Links ]
Turner, D.P. & E.H. Franz. 1985. Size class structure and tree dispersion patterns in old-growth cedar-hemlock forests of the northern Rocky Mountains (USA). Oecologia 68: 52-56. [ Links ]
Urban, D.L., R.V. O'Neill & H.H. Shugart. 1987. Landscape ecology. Bioscience 37: 119-127. [ Links ]
Wartenberg, D., S. Ferson & F.J. Rohlf. 1987. Putting things in order: a critique of detrended correspondence analysis. Am. Nat. 129: 434-448. [ Links ]
Whittaker, R.H. 1967. Gradient analysis of vegetation. Biol. Rev. 42: 207-264. [ Links ]
Wildi, O & L. Orlóci. 1990. Numerical exploration of community patterns. SPB Academic, The Hague. 124 p. [ Links ]
1Facultad de Ciencias Agrarias, Universidad Nacional de Rosario, Casilla de Correo 14, 2123 Zavalla, Argentina. FAX 54-041-257164. E-mail: barberis@cidoc.edu.ar














