Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta Médica Costarricense
versión On-line ISSN 0001-6002versión impresa ISSN 0001-6012
Acta méd. costarric vol.54 no.1 San José ene./mar. 2012
Original
Analysis and Characterization of the Pharmacotherapy of Low Molecular Weight Heparins Prescribed in Hospitalized Patients at the Hospital Clinica Biblica (Costa Rica) from March to August 2010
Gustavo Céspedes-Orozco1, José Miguel Chaverri-Fernández2,3, Jeime López-González1, Esteban Zavaleta-Monestel.3
Clínica Bíblica Hospital, San José, Costa Rica
Abbreviations: ACCP, American College of Chest Physicians; LMWH, low molecular weight heparin; CBH, Clínica Bíblica Hospital; ISHM, Integrated System for Hospital Management; PTE, pulmonary thromboembolism; VTE, venous thromboembolism; DVT, deep venous thrombosis.
1 Pharmacy interns, University of Ibero-America, Costa Rica.
2 Department of Pharmacology, Toxicology and Pharmacodependence, Faculty of Pharmacy, University of Costa Rica.3 Pharmacy, Clínica Bíblica Hospital, Costa Rica.
Correspondence:
Abstract
Aim: To analyze the prescription of low molecular weight heparins in hospitalized patients at the Clinica Biblica Hospital (private hospital in Costa Rica) based on the guidelines established by the American College of Chest Physicians (ACCP;2008).
Material and methods: This study included 1651 hospitalized patients in the period from March to August 2010 who were treated with low molecular weight heparins, 250 patients were analyzed and randomly selected. A compilation of documents and information required for each patient for the analysis was made.
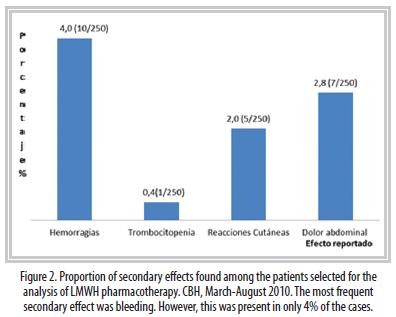
Results: A total of 43% of the hospitalized patients used low molecular weight heparins (707 patients). In 91% of cases low molecular weight heparins were used with a prophylactic purpose. 2% of patients did not need to use prophylactic heparin therapy. In 90% of cases the dose was correct. 18% of cases required dose adjustments. In 80% of the patients had clinically relevant drug interactions, 4% of patients had some form of bleeding, where 2% of cases this effect was linked to the use of low molecular weight heparins. In 9% of cases in which LMWH were used as treatment were addressed in accordance with established guidelines.
Conclusion: In the Hospital Clinica Biblica low molecular weight heparins were used according to the recommendations established by the guidelines of the ACCP despite the non-existence at the time of a hospital protocol. A pharmacotherapeutic analysis by the clinical pharmacist can provide important information to the medical doctor in order to take corrective and / or preventive actions associated with the correct use of medications. Make correct risk stratification and individualization of treatment facilitates proper implementation of drug therapy with low molecular weight heparins in this hospital where there is a high proportion of patients that may develop thromboembolic events.
Keywords: Low molecular weight heparins, Drug utilization study, Deep vein thrombosis, thromboprophylaxis, side effects, bleeding.
The type of thrombogenic substrate determines the use of LMWH for antithrombotic treatment. The main conditions where LMWH is preferred are: pulmonary thromboembolism (PTE), stroke, myocardial infarction, transient ischemic seizures and treatment or prophylaxis for deep venous thrombosis (DVT).4,7
Routine assays have demonstrated that pharmacologic prophylaxis for DVT must be the treatment of choice in most patients hospitalized for more than one or two days, after risk stratification (main indication for LMWH)7. A single daily dose of LMWH is effective and safe, and it halves the risk of DVT without and increase on hemorrhagic complications.7
LMWH is widely used in the aforementioned indications, and, therefore, it is important to be able both to evaluate the efficacy or safety of the prescription and the way it is issued and its compliance with the accepted guideline (ACCP 2008 in our case). The latter variables are quantifiable by means of medication use evaluation studies.8
Medication use evaluation studies can provide plenty of information and multiple useful answers for the improvement of the management of drugs in a hospital, in order to accomplish a more rational use of them, to reduce the cost of treatments or to improve the way health conditions are treated.8-12
The American College of Chest Physicians (ACCP) guidelines have been developed in order to assure the rational and correct use of anticoagulant therapy (including LMWH among others), by means of therapeutic proposals based on the best available scientific evidence.8
The aim of this study was to characterize and analyze the indications for LMWH prescribed to patients hospitalized in the Clínica Bíblica Hospital (CBH) and to provide an analysis of the anticoagulant pharmacotherapy (with LMWH) that those patients received in order to validate the pertinence, efficacy and safety of these drugs, their use in view of the international guidelines (ACCP 2008) and their safety through time.
Materials and Methods
This research was a retrospective, observational medication use evaluation study. The study was carried out on hospitalized patients at CBH from March to August 2010.
All patients over 18 that received LMWH were included except those who were not hospitalized for at least 24 h. The sample size was determined by a simple random probabilistic system. Daniel Wayne's 1998 9,10 formula for a known population with 95% confidence, 5% precision and an expected proportion of 50%, which maximizes the sample size, was employed, and random selection was achieved by the use of Microsoft Excel, Microsoft Office 2010.
All documents and necessary information for the study (clinical data for every patient, discharge report, nursing administration sheets, hospital's admission databases, epicrisis, clinical laboratory tests, medical notes, pharmacologic profile, among others) were gathered by means of the medical records and two in-house softwares at CBH: the Integrated System for Hospital Management (ISHM) and RECETARIO.
A comparison between the data obtained in the study and those reported in the literature11 was carried out. A multivariate analysis including patient, disease and prescribed medicine-related factors was performed. The requirements for dose adjustment for each patient were evaluated by means of their clinical laboratory test data and the use of the creatinin clearance (Cockroft-Gault) adjusted at 72 kg.12,13
The need for thrombo-prophylaxis was analyzed individually using an adaptation of the system used by the ACCP and stratifying patients in one of the four VTE risk levels according to the type of adequate surgical procedure, age and the presence of other additional risk factors the patient may have had.
The dosing for each indication was evaluated according to the literature review and what has been published by the ACCP. In order to determine clinically significant interactions we used two softwares: Micromedex ® 1.0 (Healthcare Series) by Thompson Reuters and Lexi-ComInteract ®, analyzing the validity and significance of the information they generated. For the processing of the data we used statistical analysis tools and programs such as Excel Microsoft Office 2010 and SPSS V18 (Statistical Program for the Social Sciences).
The access to and review of the medical records was authorized by the institution's Medical Direction and local Pharmacotherapy Committee (a Scientific Ethics Committee was not available at the time).
Results
A total 1,651 patients were hospitalized during the study period. Of these, 707 were given any kind of LMWH, which represents 43% of the total hospitalized patients. A sample of 250 patients was selected from this group.
Fifty-eight percent of the selected patients were women and 43% were men. The mean age of the patients was 62 ± 15 years.
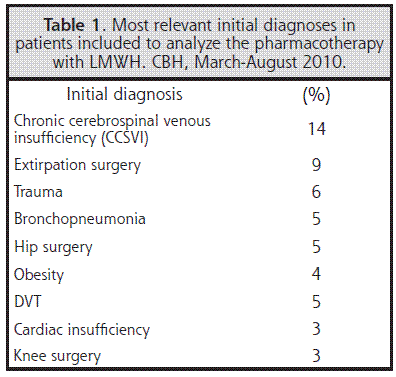
LMWH types that were used were enoxaparin (87% of the cases), bemiparin (8% of the cases), and fondaparinux (5% of the cases). Sixty-five different initial diagnoses were found, the most relevant of which are presented in Table 1.
In this hospital,
the main condition where LMWH was used was DVT prophylaxis (91% of the
cases).
In a small group of patients (remaining 9%) LMWH was used for medical
treatment
of PTE, DVT, acute coronary syndrome and/or stroke. Patients who were
treated
under these conditions were given LMWH following the established
guidelines,
with correct doses and in combination with other necessary
anticoagulant
or antiplatelet agents.
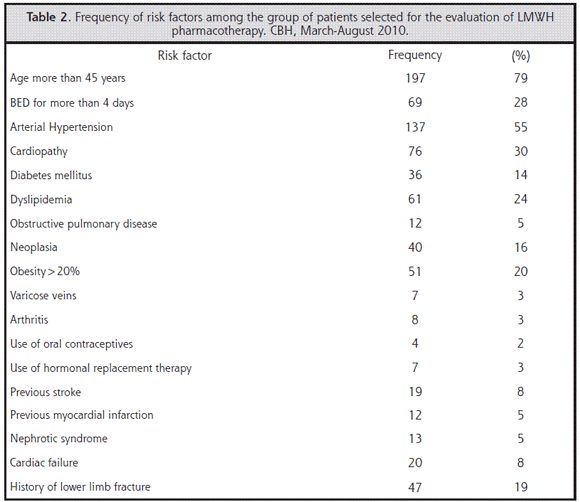
In order to be able to stratify the patients to assess the need for thromboprophylaxis as recommended by the ACCP, an analysis of individual risk factors was carried out and the most relevant are presented in Table 2.
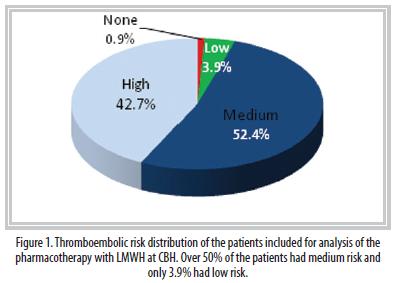
According to the risk stratification, 52.4% of the population had a medium risk of suffering a thromboembolic event. Fortytwo point seven percent had a high risk and 3.9% a low risk. Only 0.9% of the cases did not present any risk (Figure 1). Ninety-eight percent of the population included in the study and that received LMWH did require it. The LMWH treatment was changed in 4% of the patients without a clinically valid justification.
Regarding the laboratory tests performed on the selected patients, we found that 32% of the patients had prothrombin time (PT), 29% had activated thromboplastin time (aTPT), 33% had an INR, 9% had D dimer, and 79% had serum creatinin.
Discussion
The results from this study show that an important percentage of hospitalized patients (43%) received some type of LMWH, a proportion which is higher than that reported for a hospital in Madrid (36%), but similar to another report from the ENDORSE study (39%).14,15 A higher proportion of female patients receiving this medication was also identified by this study and others.14-18
Among the anticoagulant drugs analyzed in this study, enoxaparin was much more commonly used (86%), a fact also reported by Villar in 2004.17 Even though the ACCP guidelines do not specify any LMWH type, we believe that the preference towards this particular one is mainly due the higher amount indications and of clinical trial data available for it, something that may generate more confidence among prescribing clinicians.3,4,6,7,19-23
As was expected according to previous reviews, in this hospital the main condition for which LMWH was used was DVT prophylaxis (91%). Most patients had either a medium or a high risk of developing thromboembolic complications, making a high proportion of potential LMWH users. In this study, in 98% of the cases where LMWH were used as prophylaxis the treatment was correctly selected, which means that, even though there is not a current institutional protocol on this, clinicians at CBH perform an adequate risk stratification.
Either because of the presence of a secondary effect or because of a deterioration of renal function, especially among elderly patients, some cases required an adjustment of the dosage. The guidelines recommend making adjustments when the creatinin clearance is lower than 30 mL/min, and this is something that must be considered and corrected where needed. Nineteen percent of the patients required a dose adjustment, and this was performed in 86% of the cases but 22% of these adjustments were incorrect possibly because of lack of clinical data. The Department of Pharmacy must be obliged to help the clinician with the necessary dosage adjustments by means of constant pharmacotherapeutic surveillance.
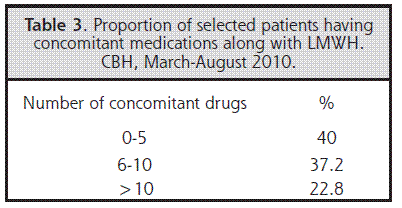
Eighty percent of the patients presented some clinically relevant pharmacologic interaction during their treatment. Adverse effects because of the concomitant use of different kinds of drugs were not observed, but this must not imply that one should not be aware of the possibility of these complications and of the clinical relevance of the data generated by these softwares and the correct use of this information prior to any intervention. It must be stressed that the information should be available before and not after a problem arises, in order to help the clinician decide following a correct risk-benefit analysis.
International guidelines underscore the fact that different types of LMWH should not be exchanged25,26 and that each one must be tested individually in clinical trials for each indication.26 Despite this, 4% of the patients had a change in the LMWH they were receiving, and in none of these cases was there a clinical or pharmacologic justification. This could be due to a personal preference from the prescribing clinician since in most cases this happened in parallel to a change of the treating clinician.
Even though one of the advantages of LMWH is that it does not require periodic laboratory test monitoring, the ACCP guidelines and much of the literature recommend that this control must be undertaken in cases where there is a concomitant use of other drugs that can increase the risk of bleeding.
In some cases, LMWH were used in (prophylactic) indications not yet approved by any international guideline. None of the studies on the safety and efficacy of the treatments for chronic cerebrospinal venous insufficiency, such as those where the patients undergo the insertion of stents or the dilation of jugular veins with balloons, has provided detailed data on this respect.29,30 Both procedures imply risks associated with elastic retraction, rupture of the vein, and development of blood clots. This does not mean that the prophylactic treatments from these surgeries will not be considered in the future, but that there is a lack of research that demonstrates that LMWH is safe and effective in cases such as palliative care of multiple sclerosis.31
Additionally,
we recommend that the hospital improve the controls for the correct
recording
of the clinically relevant data such as weight and height of each
patient
and to encourage the inclusion of all necessary information on the
medical
records among staff, in order for it to be available for future
studies.
Also, once established at the institution, it should be advisable to
keep
an up-to-date
protocol for the therapeutic management of LMWH (as defined by the
ACCP)
and to allow the Department of Pharmacy to fully engage in the process
of
pharmacologic monitoring.
References
1. Fernández A. Características de las Heparinas de Bajo Peso Molecular. Revista emergencias 2002; 14: 38-41. [ Links ]
2. Drug Information for the Health Care Professional (USP.DI) Vol. 1. 27a ed. Massachusetts: Thompson Micromedex; 2007. p 1272-1276. [ Links ]
3. Montalescot G., et al. Enoxaparin versus Unfractionated Heparin in Elective Percutaneous Coronary Intervention. N Engl J Med 2006; 355:1006-17. [ Links ]
4. Animan E., et al. Enoxaparin versus Unfractionated Heparin with Fibrinolysis for ST-Elevation Myocardial Infarction. N Engl J Med 2006; 354:1477-88. [ Links ]
5. Alba R., Catay E., Toledo R., Viana M. Heparina de bajo peso molecular versus heparina no fraccionada. Revista de postgrado de la Vía Cátedra de Medicina N°155, Marzo 2006. p 12-14. [ Links ]
6. Spyropoulos A., Frost F., Hurley J., Roberts M. Costs and Clinical Outcomes Associated with Low-Molecular-Weight Heparin vs Unfractionated Heparin for Perioperative Bridging in Patients Receiving Long-term Oral Anticoagulant Therapy. CHEST 2004; 125:1642–1650. [ Links ]
7. Hirsch DR., Ingenito EP., Goldhaber SZ. Prevalence of deep venous thrombosis among patients in medical intensive care. JAMA 1995; 274: 335-337. [ Links ]
8. Eikelboom J., Karthikeyan G., Fagel N., Hirsh J. American Association of orthopedic surgeons and American College of Chest Physicians Guidelines for Venous Thromboembolism Prevention in Hip and Knee Arthroplasty Differ: What Are the implications for Clinicians and Patients?. Chest 2009; 135: 513-520. [ Links ]
9. Wayne D. Bioestadística Base para el análisis de las ciencias de la salud. México: Ed. Limusa S.A; p 205-206 2002 [ Links ]
10. Fernández P. Unidad de epidemiologia clínica y bioestadística. Complejo hospitalario Universitario de la Coruña. Cátedra atención primaria. 1996;3: 138-140 [ Links ]
11. Geerts WH., Bergqvist D., Pineo G., Heit J., Samama C., Lassen M, Colwell C. Prevention of Venous Thromboembolism: American College of Chest Physicians Evidence Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133; 381S-453S. [ Links ]
12. Dipiro J., Talbert R., Yee G., Matzke G., Wells B., Posey LM. Pharmacotherapy: A Pathophysiologic Approach. Edition 7th. UnitedStates Of America: Ed Mcgraw-Gill; 2008.p 249- 251, 331-382, 1665. [ Links ]
13. Chisholm-Burns M., Wells B., Schwinghammer T., Malone P.,Kolesar J., Rotschafer J., Dipiro J. Pharmacotherapy principles& practice. United States Of America: Ed Mcgraw-Gill; 2008. p 83-85, 133-163. [ Links ]
14. Villar I., Urbieta E., Arenere M., López G., Marcilla F., Rabanaque M. Evaluación de la utilización de heparina de bajo peso molecular como profilaxis de tromboembolismo venoso en pacientes de medicina interna. FarmHosp (Madrid). 2004; 28 (6):402-409. [ Links ]
15. Cohen A., Tapson V., Bergmann JF., Goldhaber S., Kakkar A., et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross- sectional study. Lancet. 2008; 371: 387-394. [ Links ]
16. Donald A., Kahn S., Shrier I. Missed Opportunities for Prevention of Venous Thromboembolism: An Evaluation of the Use of Thromboprophylaxis Guidelines. CHEST 2001; 120; 1964-1971. [ Links ]
17. Molero M., Abadín J., Durán J., Sánchez A. Estudio de utilización de la profilaxis tromboembolica venosa en cirugía de cadera. RevOrtopTraumatol. 2003; 47: 129-133. [ Links ]
18. Aujesky D., Guignard E., Pannatier A., Cornuz J. Pharmacological thromboembolic prophylaxis in a medical ward. J Gen Intern Med. 2002; 17: 788-791. [ Links ]
19. Samama M., Thomas A., Darmon J., Desjardins L., Eldor A., et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med 1999; 341: 793-800. [ Links ]
20. Riestra B., et al. Análisis costo-efectividad de enoxaparina en la profilaxis de la enfermedad tromboembólica venosa en pacientes sometidos a cirugía mayor ortopédica. Farmacia Hospitalaria,Madrid. Vol. 27. N.° 4. p 210-218, 2003. [ Links ]
21. Stinnett J., Pendleton R., Skordos L., Wheeler M., Rodgers J. Venous thromboembolism prophylaxis in medically Ill patients and the development of strategies to improve prophylaxis rates. American Journal of Hematology. 2005; 78: 167-172. [ Links ]
22. Tapson V., Decousus H., Pini M., Chong B., Froehlich J., et al. Venous thromboembolism prophylaxis in acutely III hospitalized medical patients: Findings from the international medical prevention registry on venous thromboembolism. Chest. 2007; 132: 936-945. [ Links ]
23. Piazza G., Seddighzadeh A., Goldhaber S. Double trouble for 2609 hospitalized medical patients who developed deep vein thrombosis: prophylaxis omitted more often and pulmonary embolism more frequent. Chest. 2007; 132: 554-561. [ Links ]
24. Páramo J., Feliu J., Iglesias R., Ruiz E., Lecumberri R. Profilaxis del tromboembolismo venoso: recomendaciones en pacientes médicos y sistema de alarma electrónica en pacientes hospitalizados. RevMedUniv Navarra. 2006; 50(1): 17-23. [ Links ]
25. Hirsh J., Bauer K., Donati M., Gould M., Samama M., Weitz J. Parenteral Anticoagulants: American College of Chest Physicians Evidence Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133; 141S-159S. [ Links ]
26. Parras M., Tuneu L. Guía de seguimiento farmacoterapéutico sobre anticoagulación y procesos trombóticos. EspaiGràficAnagrafic, S.L. España. 2005; 10-11 [ Links ]
27. Fareed J., Ma Q., Florian M., Maddineni J., Iqbal O., Hoppensteadt D., Bick R. Differentiation of low-molecular-weight heparins: impact on the future of the management of thrombosis. SeminThrombHemost.2004;30:89-104 [ Links ]
28. Díaz R., Profilaxis de la trombosis venosa y de la hemorragia digestiva en la sepsis grave. Rev. De Medicina Intensiva, 2005; Art.C24. Vol 5, No.1. [ Links ]
29. Zamboni P., Galeotti R., Menegatti E., Malagoni A., Tacconi G., et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J NeurolNeurosurg Psychiatry 2009;80: 92–399 [ Links ]
30. Zamboni P., Galeotti R., Menegatti E., Malagoni A., Gianesini S., et al. A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency. J Vasc Surg.2009;50(6):1348-58 [ Links ]
31. Rogers, Roberts, Schloesser, Wong. Serie Radiología Clínica: Los 100 principales procedimientos intervencionistas.Elsevier España SA. 2004: 160-167 [ Links ]
Fecha recibido: 5 de
abril
de 2011 Fecha aceptado: 3 de noviembre de 2011











 texto en
texto en