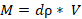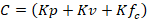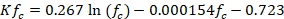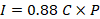Introduction
Gold mining liabilities may negatively affect the environment by contaminating soils, groundwater, and surface water with metals (Naicker, Cukrowska & McCarthy, 2003; Singh, Zeng & Chen, 2005; Pruvot, Douay, Herve, & Waterlot, 2006; Navarro, Pérez-Sirvent, Martínez-Sanchez, Vidal, Tovar & Bech, 2008) and consequently threaten the health of the people that use these resources. Human exposure to metals, such as Copper (Cu), Lead (Pb) and Cadmium (Cd), in leachates from mining wastes is associated with some diseases. The effects of cadmium exposure may occur primarily in the form of kidney damage but possibly also bone effects and fractures (Jarup, 2003). Even if the concentrations of these metals in mining waste are low, the problem persists, as the magnitude of the problem also depends on the amount of waste (Rösner & Van Schalkwyk, 2000), the environmental conditions to which they are exposed and the mobility of the metals. In tropical environments, where temperatures are generally higher than 18°C and rainfall is intense, the mobility of metals tends to increase significantly. Besides, erosion and oxidation of minerals present in mining waste create acidic conditions (Rösner & Van Schalkwyk, 2000; Naicker et al., 2003), facilitating the mobilization of metals into surface and groundwater (Naicker et al., 2003; Candeias et al., 2011; Craw, 2000).
In the past, mining activity in Costa Rica has left abandoned mining wastes, such as those found in the town of Líbano located in Tilarán, Guanacaste province (Singer, Norman, Bagby, Cox & Ludington, 1990), whose activity ceased at the end of the 1990s and currently could constitute a potential contamination risk for the San José River. At present, the degree of conjugation between factors such as the intense exposure of these wastes to erosion and the permanent oxidation process to which they are subjected is unknown. Both factors promote the removal of significant amounts of contaminating material through the formation of runoffs and leachates with low pH values (Rösner & Van Schalkwyk, 2000). The leachate from the abandoned mine is frequently enriched with toxic elements, but its movement off-site is diffuse (Kyungmin, Juhee & Seunghun, 2018).
The mining waste resulting from the physical and/or chemical extraction of the mineral of interest, primary mineral, is composed mainly of gangue minerals and secondary minerals of little economic interest. As a product of the crushing for the extraction of gold (Au) and silver (Ag), these rock wastes are formed by very fine particles and exposed to a large surface area subject to oxidation reactions (Jambor, 1994). They may contain significant amounts of calcium oxide (CaO), added during the neutralization process, and other metals resulting from the chemical reactions that took place between their mineral components and the reagents that were added during the same process (Seal II & Foley, 2002).
Mining wastes may also contain sulfides (Rösner & Van Schalkwyk, 2000) associated with deposits, which are responsible for creating acidic conditions during the formation of leachates. For example, the presence of pyrite ore (FeS2) in wastes leads to oxidation processes that cause acid drains (González, Sánchez, Márquez, Lizárraga, & Durán, 2008; Nicholson, 1994). The epithermal deposit found in Líbano of Tilaran, which is of the Sado type (Singer et al., 1990), was formed from low-sulphurization chemical fluids, which implies that they are reduced (H2S) and have a pH close to neutral. Low-sulfidation deposits may contain silver (Ag), minor amounts of lead (Pb), zinc (Zn) and copper (Cu), and associated minerals such as quartz, pyrite, and galena (Camprubí, González-Partida & Levresse, 2003).
The hazard of mining waste and the leachate will depend on the toxicity of the waste and its geo availability and bioavailability. Both are determined by the geology, concentrations of metals and metalloids, the action of biological and geochemical factors on the waste, and the presence of organic matter in the soil (Plumlee & Nash, 1995). The amount of mining waste and the vulnerability of the affected surface waters are factors that influence the hazardousness of the waste. Therefore, the main objective of this study is to assess the size of the problem. The study included quantifying the amount of mining waste, its characterization and assessment of the quality of leachate formed. Final aim was to demonstrate to what extent this environmental liability is a potential source of contamination of the surrounding surface freshwater ecosystem.
Methodology
Study area
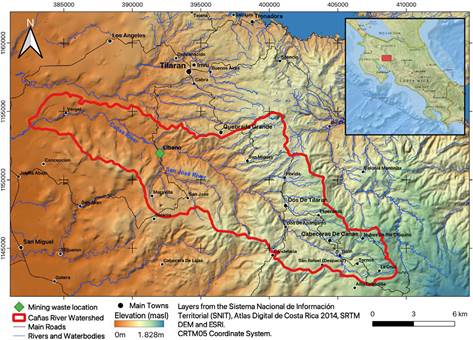
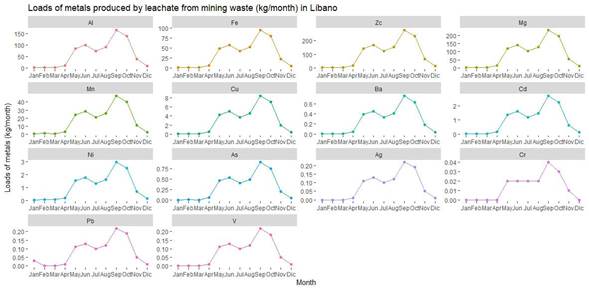
The old "Esperanza" mine is in Líbano, 8 km southeast of Tilarán in Costa Rica. Industrial and artisanal mining activity in this area took place between 1907 and 1986 with the exploitation of 5 epithermal veins (Castillo, 1997). At present, the mining liabilities resulting from this activity continue to be abandoned in Líbano, accumulated at 296 meters above sea level on the banks of the San José River, an important tributary (base flow 0.03m3/s) of the Cañas River sub-basin that flows into the Tempisque-Bebedero Basin, North Pacific Region of Costa Rica (Figure 1). This is an area dominated by alluvial and colluvial surface deposits, of recent age, which also include landslide deposits, mudstones, swamp and beach deposits (Arce, 2004). Líbano is located at the confluence of several mountain streams at risk of detrital flows and flooding (Arce, 2004). The geology of the area is dominated by andesitic lava, tuff and lapilli tuff of the Aguacate Group, which present pervasive propylitic alteration (Cooke, 1987).
The annually average rainfall in this area is 1805, 1 mm, and the highest rainfall occurs in September and October (ICE, 2015). Six ranges of average temperatures predominate; the lowest being 17 ° C and the highest 27 °C (IMN, 2010).
Sizing and characterization of mining waste
Volume and sampling of mining waste
A tape measure was used to measure the area of the mining waste pile in greater detail. The approximate heights of the mound were measured with sinkers from the surface to the base of the San José River. The average heights (H) and surface areas were used to estimate the volume (V) of the waste, assuming a rectangular base.
The area of the upper part of the waste was divided into two plots (Lib A and Lib B), each measuring approximately 3.909 m2. In a triangular arrangement (3 m distance from one point to another), in the center of each plot, three boreholes were drilled, each 1 m deep, from which a composite sample of ~ 450 g was obtained (150 g extracted every 30 cm deep). The three samples obtained from each plot were separately homogenized by hand to obtain two representative samples (Lib A and Lib B). Both samples were placed in plastic bags for laboratory analysis and kinetic testing.
Minerals in mining waste
X-Ray Diffraction Analysis (XRD) was performed on Lib A and Lib B samples with an X-Ray Diffractometer (Bruker, D4 Endeavor) to identify the minerals present in the mine waste. To identify the minerals from the crystalline powder diffraction diagrams, diffraction patterns contained in the JCPDS (Joint Committee for Powder Diffraction Sources) database of the ICDD (International Center for Diffraction Data,1997) (www.icdd.com) were used.
Metal concentration in mining waste
Before obtaining the composite samples Lib A and Lib B, 1.5 g of each of the 3 samples obtained from each plot were analyzed following the procedure 3030E APHA (1995), for total metal concentrations. Samples were digested with a mixture of 1.5 ml of HNO3 (69%) and 4.5 ml of HCl (37.5%) at 85 oC. The digests were analyzed by atomic absorption spectrophotometry (Perkin Elmer, AAnalyst 800) with flame technique for Cu and Mn, with graphite furnace for As, Al, Cd, Cr and Pb, and by cold steam (Perkin Elmer, MHS 15) for Hg. During the analyzes, control standards were used with certificates drawn to the NIST, read at the beginning and at the end of each run; these control standards were also used to prepare fortified samples, with both values obtaining recovery percentages of 90-110%. The sample blank used in this analysis corresponds to the one that was subjected to the same digestion as the waste samples. A calibration blank was also used in the laboratory. Calibration curves were performed with a total of 6 to 8 standards.
2.2.4 Particle density and estimation of the amount of mining waste
The particle density (dρ; kg/m3) of the mining waste was determined on 5 replicates of 200 g (dry weight) of a mixture of Lib A and Lib B samples.
The total mass of material (M: kg) was estimated as:
V: volume (V) of mining waste, dρ: particle density (dρ: kg/m3)
Quantity of metals present in mining waste
The amount of mass (ton) of each metal present in the mining waste was estimated using the average of the concentrations (mg/kg) determined in the three sub-samples of the composite samples Lib A and Lib B (Cmetal), and the total amount of mining waste (M: kg).
Leaching and loading of contaminants
Wet cell kinetic test
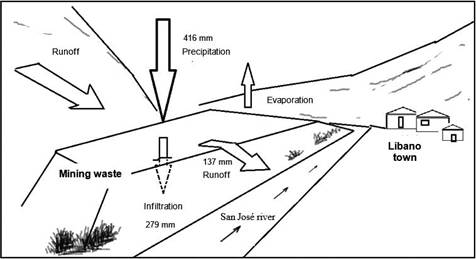
To assess what effect oxidation has on the waste material, a waste sample was subjected to the environmental conditions of the site. A kinetic test was carried out in two cycles, lasting 7 and 6 days each, using a moisture cell according to Sobek, Schuller, Freeman and Smith (1978). Using this test also information was obtained on the resulting acidic conditions and the quality of the leachate produced.
The moisture cell (Figure 2) was constructed with a 60 cm long, 7.6 cm internal diameter PVC pipe. Three hoses were fitted to this pipe: one at the top for water intake and air outlet, one at the bottom for the output of the leachate produced, and a third located laterally at the bottom for the intake of pressurized air (100 lpm). An oxygen cylinder incorporated into the system using a flow meter and a humidity trap was used as an air source.
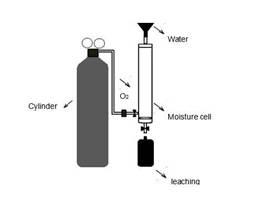
Figure 2 Moisture cell for the kinetic test used to determine effects of oxidation on leaching quality produced by mining waste materials
On the first day of testing, 200 g of a composite sample obtained from the mixture of Lib A and Lib B samples was placed in the cell. The lateral dry air inlet and the upper air outlet were left open during the first three days of each cycle, the inlet for humid air on the following three days. The air was made to flow from the bottom of the cell upwards through the entire sample. On the last day of each cycle, the sample was leached with 450 ml of distilled water (corresponding to 98.7 mm/m2) and the respective leachates, Lix 1 and Lix 2, were collected and stored at 4°C. Both leachates were measured for dissolved oxygen, pH and electrical conductivity using multi-parameter equipment (OAKLON, 600 Series) and electron transfer (ORP) (Thermo scientific ORP, H00862 Series). In addition, total Fe and SO4 2+ concentrations were determined by visible ultraviolet spectrophotometry (APHA, 2005).
A composite sample obtained from Lix 1 and Lix 2 was digested with 1:1 HCl (37.5%) and HNO3 (69%) for the analysis of metals (Al, As, Ba, Cd, Cu, Cr, Fe, Mg, Mn, Hg, Ni, Ag, Pb, Zn, and V). This analysis was performed through inductively coupled plasma optical emission spectrometry (ICP-OES Agilent Series 715 Radial) following Standard Methods (APHA, 2005). All samples were read in duplicate, using a control for each measurement range.
Mine waste infiltration capacity
Infiltration tests were conducted at three sites representative of the entire waste area, at 30 m distance from each other, following Porchet Method or Well Permeameter Method (USBR 7300-89). An auger was used to make three holes 45 cm deep. Each hole was saturated with water as many times as necessary until the level slowly dropped. The hole was then filled again with water, and with a tape measure and a millimeter ruler the decrease of the water (cm) was measured every minute for 10 min, and then every 5 min until 1 h was completed.
The infiltration capacity (f: cm/min) of the mining waste was estimated from the reduction rate of the water level and the diameter of the hole.
Metal leaching from mining waste
The amounts of water (mm/month) infiltrating in the mining waste and draining superficially and/or evaporating were determined with the infiltration capacity (f: cm/min) and the equations proposed by Schosinsky and Losilla (2000). The monthly average precipitation rates recorded from 2001 to 2015 at the meteorological station in Líbano (Table 1) were used in the estimates of leachate generated from mining waste.
Table 1 Monthly average rainfalls, P (mm/month), from 2001 to 2015, recorded at the weather station of Líbano in Costa Rica(ICE, 2015)
| Month | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sept | Oct | Nov | Dec |
| Rainfall | 5.3 | 7.6 | 6.7 | 26.2 | 212.5 | 250.6 | 183.9 | 228.3 | 415.7 | 349.3 | 97.8 | 21.2 |
Source: Service Center for Basic Engineering Studies - Hydrology, ICE, (2015).
The infiltration coefficient (C) (Schosinsky & Losilla, 2000) was estimated with Equation 2:
Where: K p: Fraction that infiltrates by slope effect (something flat = 0.15); K v: Fraction that infiltrates by vegetation cover effect (no vegetation = 0); Kf c: Fraction that infiltrates by soil texture.
The basic potential infiltration (fc: mm/day) (Schosinsky & Losilla, 2000) was determined in the test on the infiltration capacity (f: cm/min) of mining waste. Kf c is related to f c as:
The amount of rain (mm/month) infiltrating was estimated as:
Where: I: infiltration (mm/month); C: infiltration coefficient; P: monthly precipitation (mm/month)
Pollutant loading
The load of metallic contaminants (CC: kg/month) in the leachates that originate from the mining waste was estimated considering the concentrations of the leached metals (kinetic test), the infiltrated precipitation that corresponds to the flow of the leachate per month (l/m2 s) and the total area of the mining waste (m2) according to Equation 5:
CC=Q×A×Cmetal×2.592 (E. 5)
Where: Q: leachate flow (l/m2 s); A: mining waste area (m2); C metal : metal concentration (mg/l); 2.592: conversion factor (kg/month) (modified from Roldán & Ramírez, 2008).
Results
Sizing and characterization of mining waste
The mining waste in Líbano is in a rectangular collection pile. This allowed the physical dimensioning of the waste: a surface area of 7820 m2, a volume of 54738 m3, an estimated total amount of 106 000 tons and an apparent density (ρa) of 1936 ± 39 kg/m3.
The mineralogical composition of mining waste in Líbano qualitatively determined by diffractograms in DRX tests was represented by primary non-metallic gangue minerals, such as quartz (SiO2), mostly medium-neutralizing minerals such as illite/smectite/montmorillonite (KAl3Si3O10 (OH)2) and chlorite ((Mg5Al) (Si, Al) 4O10 (OH)8), primary metallic gangue minerals such as pyrite (FeS2), and secondary minerals such as gypsum (Ca(SO4)(H2O)2) and magnetite (Fe3O4). Qualitatively, the minerals that were present mostly are quartz and gypsum, and in smaller proportion pyrite and chlorite and others.
The mining wastes showed yellow-brown coloration on the surface while inside the waste the coloration changed to grey. In this place there was little vegetation and superficially the soil was not compacted. In addition, there was evidence of the entry of people and animals to this site (Figure 3).
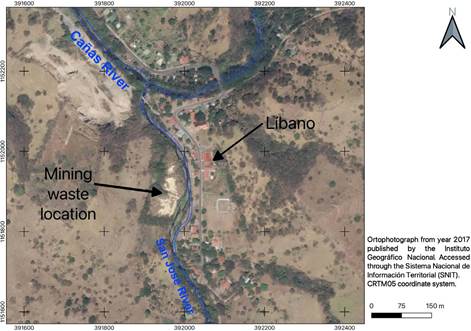
Figure 3 Mining liabilities exposed to a current condition of oxidation and erosion near the San José river in Líbano, Costa Rica.
The concentrations of metals in the mine waste in Líbano are shown in Table 2.
Table 2 Concentrations (mg / kg) and quantity of metals (tons) in mining waste in Líbano, Costa Rica.
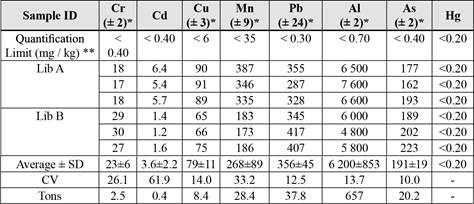
* Value of the uncertainty of the analytical measurement; SD: Standard Deviation; CV: Coefficient of Variation.
** Quantification Limit values are those obtained by LASEQ laboratory, UNA.
(+) Reference methods are those used by the laboratory for spectrophotometric measurements.
Leaching and pollutant loading
Table 3 shows the results of the physical-chemical analysis performed on the leachate from the sample of mining waste subjected to oxidation and Table 4 of the metals analysis performed on the leachate during the kinetic test. The measured parameters show the chemical processes that occurred due to oxidation in mining wastes during the transition from dry-rainy season (Lix 1) and rainy season (Lix 2): the state of the acidity of the medium (pH), the dissolution of the ions (EC), the oxidizing condition of the medium (ORP) and the metal concentrations that are released to the medium by the oxidation of the pyrite (iron and sulfates).
Table 3. Characteristics of leachates obtained in the wet cell kinetic test on mining waste samples from Libano, Costa Rica.
| Sample | pH | Electrical Conductivity (EC) (µs/cm) | Redox Potential (ORP) (mV) | Iron (mg/l) | Sulfates (mg/l) |
| Lix 1 | 4.16 | 3 620 | 275 | 1.61 | 3 029 |
| Lix 2 | 4.41 | 466 | 219 | 21.5 | 267 |
Table 4. Metal concentrations in leachates of composite samples (Lix 1 + Lix 2) of mining waste from Libano, Costa Rica.
| Metal | Concentration (mg/l) | Detection Limit (mg/l) |
| Aluminum (Al) | 75.9 | 0.013 |
| Arsenic (As) | 0.411 | 0.006 |
| Barium (Ba) | 0.346 | 0.005 |
| Cadmium (Cd) | 1.23 | 0.002 |
| Copper (Cu) | 3.85 | 0.005 |
| Chrome (Cr) | 0.019 | 0.005 |
| Iron (Fe) | 44.0 | 0.014 |
| Magnesium (Mg) | 107 | 0.014 |
| Manganese (Mn) | 21.8 | 0.005 |
| Mercury (Hg) | < LD | 0.005 |
| Nickel (Ni) | 1.37 | 0.005 |
| Silver (Ag) | 0.101 | 0.022 |
| Lead (Pb) | 1.03 | 0.019 |
| Zinc (Zn) | 127 | 0.004 |
| Vanadium (V) | 0.100 | 0.022 |
<LD: Less than the detection limit of the method
The estimated infiltration capacity (f) of mining waste was 239 mm/day. This low infiltration capacity resulted in the formation of runoff. Based on the results of the infiltration measurements and using the historical precipitation, the behavior of the infiltration rate in the mining waste was estimated (Figure 4). Additionally, the contaminant loads in the leachates generated from the mining waste were estimated (Table 4).
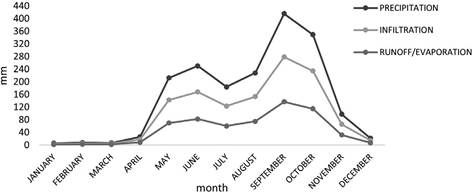
Figure 4 Seasonal patterns in precipitation, infiltration and superficial run-off on mining wastes in Líbano, Costa Rica (2001-2015).
Management of mining liabilities
The location, environmental condition, metal content and quantity of mining waste have probably affected the San José River for more than 20 years due to contamination by toxic metals. It is necessary to take into account the processes that occur in this mining liability, mainly during rainfall (Figures 3and6), to propose management alternatives.
Discussion
Sizing and characterization of mining waste
The amount of waste resulting from gold and silver mining in Líbano is ~106 000 tons. This material has a composition related to the geology of the Sado type epithermal deposit of La Esperanza mine and a particle size of less than 150 μm (sand texture). This characteristic results in a large surface area exposed to weathering and oxidation (Figure 3), and consequently in the release of metals into the environment (Plumlee & Nash, 1995), as demonstrated by leachate (wet cell) samples that evidence the ease with which metals are released from mine waste (Tables 3and4).
The yellow-brown coloration observed on the surface of the wastes indicates a degree of advanced oxidation (Figure 3), which was confirmed by the ORP and pH values (Table 3) of the leachates indicating an oxidized environment. These conditions lead to acidification and consequently facilitate the release of metals. However, a change in the coloration to grey was observed inside the waste, which is associated with a low degree of oxidation (Corrales & Martin, 2013).
Due to the high capillarity generated by the fine particles and the degree of compaction of the waste, precipitation drains slowly by gravity through the waste material causing the layers below the dry surface (~ 2 m deep) to remain permanently saturated with water. This condition makes the waste texture loose and soft, losing resistance during seismic liquefaction, and making it susceptible to water and/or wind erosion (Seed, Tokimatsu, Harder & Chung, 1985). However, the density of the debris, conferred by the presence of minerals such as gypsum, gives it certain stability, preventing extreme erosion.
Gypsum in the mining wastes may contribute to reducing the dispersion of clays by the mutual repulsion between highly hydrated ions, such as sodium and magnesium. These ions are attracted to the surface of the clay particles promoting the flocculation of the soils, which is leading to the formation and stabilization of the waste structure. This allows greater infiltration and percolation of water and air (Chen & Dick, 2011).
Although the mining wastes were previously subjected to reaction with calcium oxide, the presence of pyrite and its contact with air and/or dissolved oxygen in the percolating water, have led to oxidation (Ritchie, 1994; Robertson, 1994). This natural process occurs slowly producing sulfuric acid and ferric sulfate, which generate acidic drains. These conditions also promote the leaching of metals (Table 4), which consequently presents a risk of contamination of the San José and Cañas Rivers surrounding the area, (González et al., 2008). In order of highest to lowest concentration, the metals present in the leachates were Zn>Mg>Al>Fe>Cu>Ni>Cd>Pb>As>Ba>Ag>V>Cr>Hg (Table 4). The high concentrations of aluminum and manganese can be explained from their presence as natural components of the clays that are present in the waste material. Mercury was present in the waste only at trace levels (Table 4), suggesting that this element was not used in the gold recovery process.
The CV range (Table 2) is an indication of homogenous of the metals distribution, except for cadmium, which shows values greater than 60%. Although the concentrations (tons) of lead and arsenic are higher than those of cadmium in mining waste (Table 4), higher concentrations of cadmium are leached into the river, which represents a greater mobility of this metal in water.
The high concentrations of arsenic in mining waste (Table 2) limit the use of this area for agriculture since it exceeds the guide value for concentration (12 mg/kg) in soils intended for agriculture (CCME, 2014). Edible terrestrial plants take up arsenic through the roots or through their leaves when the arsenic is airborne. The presence of arsenic and lead (Table 2) in the waste materials limits its residential use, as the concentrations of both elements are above the regulatory values of 70 and 140 mg/kg, respectively (CCME, 2014).
The presence of mining waste in the town of Líbano and its use to fill in crop areas has implied the alteration of the natural conditions of the river, also because of the high concentrations of metals (Table 2). The plants that grow in these fillings show abnormal features like different coloring and morphology than plants of the same species grown in soil free of mining waste material.
Leaching and loading of contaminants
Figure 6 shows the dynamics of the processes that interact with mining waste and that condition the release of metals into surface waters in Líbano.
One of the geochemical processes that take place in mining waste during the rainy season and that affects the concentration, distribution or structure of the chemical compounds present, is pyrite oxidation. This oxidation occurs abiotically mainly in the surface layers of the wastes, which have a pH above 4.0. The balance between water and oxygen (Reaction R. 1) could control this oxidation.
The Lix 1 sample (Table 3) represents the leachate that is produced in the transition from the dry to the rainy season, during the first rainfall. The Lix 2 sample is indicative of the leachate that is obtained from mining waste that is humid and oxidized during the rainy season (Table 3). Pyrite oxidation can be more rapid during the dry season (Belzile, Chen, Yu-Wei, Mei-Fang & Yuerong, 2004) with the increase in temperature acting as a catalyst; therefore, it occurs mostly at the surface where air diffusion favors oxidation.
The pyrite oxidation (Reaction R. 1) produces sulphuric acid, expressed as the concentration of sulfates and the Fe2+ ion (Table 3) in solution. This condition causes a higher acidity (pH ~4) and consequently the release of metals to the environment (Table 4).
2FeS2 (s) + 2H2O + 7O2 → 4H+ + 4SO4 2- + 2Fe2+ (R. 1)
In the second stage (Lix 2 sample), moisture in the cell, oxidation, and acidity, allow Fe2+ to oxidize to Fe3+ (R. 2). This is due to buffering of the acidity produced by the presence of neutralizing minerals in the waste. The pH may allow a certain amount of Fe3+ to precipitate producing solid ferric hydroxide (R. 3), which occurs at pH>4 (Table 3). The neutralizing minerals present dissolve under these conditions and react with pyrite, which increases the pH a little, but decreases the ORP potential and the solubility of the sulfates, allowing the formation of gypsum (Table 3).
4Fe2+ + O2 + 4H+ → 4Fe3+ + 2H2O (R. 2)
Fe3+ + 3H2O → Fe (OH)3 (s) + 3H+ (R. 3)
Since the low pH in the waste allows the dissolution of iron (Fe2+) (R. 1) (Rose & Cravotta, 1998), it may be present in higher concentrations in the material that is transported by leaching or runoff into the San José River. These pH conditions also favor the dissolution of other minerals that results in increased leachate conductivity (Table 3).
The sulphuric acid released in the pyrite oxidation reaction (R. 4) also solubilizes the illite and chlorite, allowing the waste to be neutralized (Craw, 2000). This process provides some aluminosilicates, precipitates the sulfates as gypsum, releases Ca2+, Mg2+, K+, and HCO3 - ions and contributes to raising the pH (Langmuir, 1997). This favors the adsorption of metal ions on the surfaces of iron oxides present, forming metal oxyhydroxides and preventing their release into the environment (Corrales & Martin, 2013). However, the neutralizing capacity of these minerals (aluminosilicates) is of lower intensity due to their low rate of weathering concerning carbonates (Plumlee, 1999).
2KAl3Si3O10 (OH)2 + 2H+ + 3H2O ↔ 2 K+ + 3Al2Si2O5 (OH)4 (R. 4)
As previously stated, the wastes were treated with calcium oxide (CaO) to eliminate cyanide (NaCN) and buffer acidity by oxidation. However, the oxidation of pyrite by oxygen and water generates four moles of protons (H+), so neither the lime nor the natural neutralizing minerals present are sufficient to completely precipitate the sulfuric acid in the form of gypsum and neutralize the surface oxidation processes that have continued for years. At low pH, the metals are displaced from the oxides by protons (H+) (Triverdi & Axe, 2000). Consequently, they are released into the precipitating water involving chemical association and transport processes such as leaching and runoff. This suggests that this area is the main source of metal contamination in the San José and later Cañas rivers.
The content of some metals in the leachate was not related to their concentration in the waste. Since, for example, the concentration of chromium in the leachate was about 60 times lower than that of cadmium, even though the amount of chromium (in tons) in the waste was 6 times higher than that of cadmium. Arsenic concentration in the composite leachate was 9 times lower than that of copper, but its content in tons in the waste was more than 2 times higher than that of copper (Tables 2and4).
Aluminum and manganese were metals found in large quantities in waste (Table 2) and showed high tendency to leach (Figure 5). This may be explained from their being part of the chemical structure of neutralizing clays or minerals, which are dissolved upon pyrite oxidation and therefore more easily released. In addition, the gypsum present in the wastes may react with the soluble aluminum contributing to its release (Chen & Dick, 2011).
Rainfall peaks in September (Figure 4) resulted in increased infiltration (279 mm) and surface runoff/evaporation (137 mm). During this month, the water that precipitated in the mountain (Figure 6) adjacent to the waste zone (cut off water from the San José river basin) also formed part of the volume that leached or ran off from this zone. In the months with less precipitation, the largest volume of water infiltrated and the remaining water evaporated (Figure 4). These processes occurred mainly at the surface level because the innermost layers of the waste were saturated, therefore, the water that infiltrated travelled according to the slope of the land, saturating the lower parts of the waste zone until it reached the San José river (Figure 6).
The metals that were released were transported from the mining waste area to the river by the infiltrated water, as well as probably by the water that ran off the surface. The material gypsum did not contribute to the acidity of the environment. Its presence is associated with the ubiquity of calcium and sulfate; however, the latter is highly soluble (241 g/l), so it dissolved easily during rainfall contributing with the dragging of materials from the waste zone surfaces to the river (Seal II & Foley, 2002). Another way in which contaminants were also dragged from the mining waste was when the waters of the San José river contacted the waste during the rainy season, dissolving the material and incorporating the metals and other chemical compounds present.
Management of mining liabilities
Líbano is located in a seismic zone affected by the Cañas fault, with the danger of landslides and flooding (Arce, 2004), a condition that could cause mining waste to suffer changes in its structural properties, related to the behavior of its geotechnical characteristics in terms of stability, infiltration, and deformation. Therefore, the management of this mining liability should be done through the design of structures that contribute to keeping the waste stable and free of oxidation and reduce the infiltration of water in the mountain of wastes to avoid leaching metals to rivers.
Conclusion
The presence of quartz minerals, gypsum, pyrite, and chlorite gives the mining waste structural stability. However, weathering constantly erodes its structure while facilitating the permanent oxidation of its surface layers. Acidic conditions are created that favour the release of metals such as Pb, As, and Cd to the water percolating through the waste material in the dry-rainy transition period and dry season. Thus, the large amount of waste and its high concentrations of metals make these mining liabilities in Líbano a source of pollution for the surface water, sediments and benthic macroinvertebrates of the San José river. The high concentrations of Pb and As contained in the mining liabilities make these soils unsuitable for agricultural or residential purposes, due to the risk they pose to the health of man and the environment. Management alternatives at this site should seek to physically and chemically stabilize the structure and prevent surface oxidation.