Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Odontología Vital
versão On-line ISSN 1659-0775versão impressa ISSN 1659-0775
Odontología Vital no.39 San Pedro, Lourdes de Montes de Oca Jul./Dez. 2023
http://dx.doi.org/10.59334/rov.v2i39.552
Article
Stomatological management of anticoagulated patients undergoing oral surgery: a narrative review
a. Cirujano Dentista, Universidad Finis Terrae, Santiago, Chile ORCID: https://orcid.org/0000-0002-1956-7600 / jpcancinog@miuandes.cl
b. Cátedra de Cirugía, Facultad de Odontología, Universidad Andrés Bello, Santiago, Chile ORCID: https://orcid.org/0000-0002-1672-9205 / d.fonsecaescobar@gmail.com
c. Cátedra de Cirugía, Facultad de Odontología, Universidad Andrés Bello, Santiago, Chile ORCID: https://orcid.org/0000-0001-6221-319X / fdo.parada.f@gmail.com
Patients undertaking oral anticoagulant treatment may experience alterations in different stages of hemostasis, which lead to medical/surgical implications and considerations during their care. Currently, there is no consensus regarding the dental management of these patients, as they go through surgical procedures.
This leads to clinical protocols that follow numerous approaches, such as reducing the pharmacological intake of the anticoagulant, replacing it with heparin, and maintaining the controlled treatment.
Objective:
To establish the stomatological management of the patient undergoing oral anticoagulant treatment through an in depth review of the literature.
Materials and Method:
A manual bibliographic review search of articles indexed to the PUBMED and EBSCO databases corresponding to the words “oral surgery”, “oral bleeding”, “anticoagulants” and “dental management” was performed.
Regarding the inclusion criteria: bibliographic reviews, observational studies, clinical trials, guidelines, systematic reviews, and meta-analyses published between November 2005 and 2022, in English or Spanish, were considered.
Conclusion:
There are multiple protocols for the care of the anticoagulated patient who will undergo a minor oral surgery procedure. It is important to reflect on the anticoagulant used, the reason for it, its supervision, the surgical procedure that will be undertaken by the patient, and both intraoperative and postoperative hemostatic measures to be implemented.
After analyzing the above, it is noted that reducing the intake of the drug to perform the surgical procedure may be harmful to the patient and to the clinician, therefore it is suggested to maintain the antithrombotic treatment and carry out a correct medical/surgical management.
Keywords: Oral surgery; anticoagulants; dental care; oral hemorrhage.
Introduction
In the face of certain cardiovascular pathologies, the risk of suffering a thromboembolic event increases.
The use of oral anticoagulant therapy in primary and secondary prevention has been shown to reduce the risk of cardiovascular accidents and morbidity in this type of patient (Gazit et al., 2016).
Atrial fibrillation is the most frequent indication for receiving this treatment, with at least 3 to 6 million people affected by this pathology in the United States, with a projection of these values up to 6 to 16 million individuals in 2050.
In Europe, the prevalence of atrial fibrillation in 2010 was 9 million individuals older than 55 years, and a projection of 14 million people affected in 2060 was estimated (Di Carlo et al., 2012; Kornej et al., 2020).
Regarding the patients affected with this disease, 78% are under treatment with oral anticoagulants, and the number is growing due to the incorporation of new, safer drugs with a broader therapeutic range (Grymonprez et al., 2022).
Although most dental treatments are low risk, patients with impaired coagulation require special care to assess the risk of bleeding they present and thus prevent and/or manage any type of unfortunate event (Costa-Tort et al., 2021). Given this exponential growth in the use of chronic oral anticoagulants, this work aims to establish the stomatological management of patients undergoing oral anticoagulant treatment through an in-depth review of the literature.
Hemostasis
Hemostasis is a complex process that involves the action of platelets, coagulation factors (Table 1), and endothelium at the site of vascular injury.
It culminates in the blood clot formation, which fulfills the function of preventing or limiting bleeding. Its stages correspond to: i) primary hemostasis, ii) secondary hemostasis, and iii) fibrinolysis (Guerrero & López, 2015).
Primary hemostasis begins almost immediately when the vascular lesion occurs. In this process, platelets and the vascular wall mainly interact to limit and stop vascular extravasation in the capillaries, arterioles, and venules. In this stage, as a first instance, a vascular vasoconstriction occurs followed by the recruitment and activation of platelets, which adhere to the injured vessel, forming the platelet plug. This process is primarily mediated by the prostaglandins thromboxane A2 and prostacyclin and von Willebran factor (Gale, 2011).
Secondary hemostasis, mainly coagulation factors interact with each other, where exposure of tissue factor at the damaged site binds and activates factor Vll, starting a cascade of reactions that culminates in the generation of thrombin.
This anchors circulating fibrinogen in soluble fibrin, creating a fibrin mesh, which will reinforce the platelet plug (Hatton et al., 2013).
Finally, in fibrinolysis, the fibrin networks present in the fibrin clot are degraded by inhibiting the formation of plasmin, inhibiting tissue plasminogen activator through the release of plasminogen activator inhibitor by endothelial cells. Plasmin degrades the fibrin polymer into small fragments, which are eliminated by macrophages (LaPelusa & Dave, 2022).
Materials and method
A manual bibliographic review search of articles indexed to the PUBMED and EBSCO databases corresponding to the words “buccal surgery”, “oral hemorrhage”, “anticoagulants” and “dental care” was performed.
Regarding the inclusion criteria, bibliographic reviews, observational studies, clinical trials, guidelines, systematic reviews, and metaanalyses published between November 2005 and 2022, in English or Spanish, were considered.
From this search strategy, a total of 322 articles were obtained, of which 274 were excluded (duplicates, text not available, etc.). It resulted in a total of 44 articles for this literature review (Figure 1). The information collected was thoroughly reviewed in its entirety.
Coagulation cascade
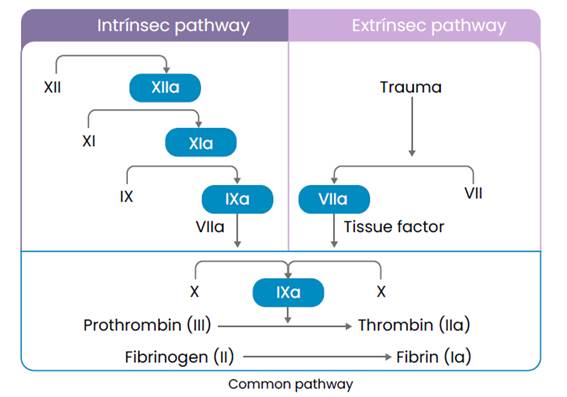
The classic model of coagulation was described by Davie and Ratnoff in 1964 and separates the cellular and humoral phases, giving prominence to the series of enzymatic reactions that occur within secondary hemostasis (Kumar et al..., 2015), which begin through the intrinsic pathway that begins after vascular damage, with the exposure of negatively charged surfaces that interact with Hageman factors (FXII), antecedent plasma thromboplastin (FXI), prekallikrein (PK) and high molecular weight kinogens (QAPM) and the extrinsic pathway which begins with the formation of the Extrinsic Tenase complex made up of tissue factor (TF), circulating factor VIIa, Ca++ ions and phospholipids (Smith et al., 2015).
These two pathways converge in fibrin activation, culminating in the deposition of an insoluble clot (Figure 2).
The formation of this clot involves enzymes (activated coagulation factor), a substrate (an inactive form of pro-enzymatic coagulation factor), and a cofactor (Onishi et al., 2016).
Table 1 Biochemical characteristics and function of coagulation factors (Adapted from Guerrero et ál., 2015)
| FACTOR | PLASMA CONCENTRATION (MG/DL) | HALF LIFETIME (HOURS) | FUNCTION |
|---|---|---|---|
| XI | 0,4-0,6 | 45-80 | In its activated form it is the intrinsic FIX activator. |
| XII | 1,5-4.5 | 50-70 | Intrinsic pathway initiator |
| PREKALLIKREIN | 1,5-4,5 | 36 | Precursor of kallikrein |
| HIGH MOLECULAR WEIGHT QUININOGENS | 8-9 | 144-156 | Cofactors in the activation of FXI and FXII prekallikrein. |
| II | 10-12 | 60-72 | Inactive precursor of thrombin |
| VII | 0,05-0,06 | 4-6 | Together with Tissue Factor initiates the extrinsic pathway. |
| IX | 0,4-0,5 | 18-25 | Inactive precursor of thrombin |
| X | 0,7-1,2 | 24-40 | Together with tissue factor initiates the extrinsic pathway. |
| PROTEIN C | 0,39-0,59 | 8-14 | In its active form, it is the enzyme of the intrinsic tenase complex. |
| PROTEIN S | 2,5 | 40-60 | PCa cofactor |
| PROTEIN Z | 0,22 | 60 | Increases the inhibition of FXa by Protein Z Inhibitor. |
| V | 0,4-1,4 | 12-36 | Prothrombinase complex cofactor |
| VIII | 0,5-1 | 8-12 | Intrinsic tenase complex cofactor |
| THROMBOMODULIN | 0 | Thrombin cofactor | |
| TISSUE FACTOR | 0 | Initiates extrinsic pathway by binding to FVIIa | |
| FIBRINOGEN | 200-400 | 90 | Precursor of fibrin |
| XIII | 1-2 | 168-288 | Transaminase that crosslinks fibrin |
| ANTITHROMBIN III | 15-20 | 68 | Serpin inhibits thrombin and factors VIIa, IXa, Xa, Xia, XIIa and kallikrein. |
| HEPARIN COFACTOR II | 6,1-8,2 | 60 | Thrombin-inhibiting serpin |
| PROTEIN C INHIBITOR | 0,5 | 23,4 | Serpin inhibits PCa, thrombin, kallikrein, FXIa, FXIIa and the C1 component. |
| Z PROTEIN INHIBITOR | 0,1-0,16 | FXa and FXIa-inhibiting serpin | |
| TISSUE FACTOR PATHWAY INHIBITOR | 0,006 | 1-2 | Kunitz-type inhibitor of TF/FVIIa/FXa and PS/FXa complexes. |
This process is calcium-dependent, which binds to a y-carboxylated glutamic acid residue present in factors II, VI, IX, and X. This reaction is dependent on Vitamin K, where its reduced form is essential to activate the aforementioned factors. Thus, Vitamin K is an important pharmacological target for anticoagulant drugs, since deficiency of this cofactor prevents blood coagulation (Ufer, 2005).
Cellular model of coagulation
Described by Hoffman and Monroe in 2001, it explains the in vivo behavior of coagulation and replaces the cascade hypothesis, giving prominence to the cells that participate in this process, which are capable of directing hemostasis, and to the importance that factor VII activity acquires. It consists of three phases, called the initiation, amplification, and propagation phase (Hatton et al., 2013; Kumar et al., 2015). This model will not be delved into, since it is not the objective of this work.
Coagulation tests
The control of the anticoagulated patient is carried out through various hematological tests, which can be classified into quantitative and qualitative, within the quantitative, we find:
Platelet count: Usually correlates with bleeding tendency. The normal count is 150-400,000 platelets/mm3 (Gomez et al., 2021).
Prothrombin time (PT), Evaluates the function of proteins of the extrinsic pathway (Factor VII, X, V II, and fibrinogen). The time it takes for the fibrin plug to form is measured. Normal values: 10-13 seconds (depending on the type of prothrombin used and the clot detection method) (Winter et al., 2017).
Activated Partial Thromboplastin Time (aPTT) Measures the function of intrinsic pathway proteins (Factor XII, XI, IX, VIII, X, V, II, and Fibrinogen). The time it takes for the fibrin plug to form is recorded. Its normal value is 25-35 seconds (Little et al., 2017).
Thrombin time (TT): The time it takes to coagulate a plasma when thrombin is added. It can be found altered in the presence of heparin and inhibitors of thrombin formation. Its normal value is 12 to 19 seconds (Winter et al., 2017).
“International normalized ratio (INR). It represents the standardization of the PT. A value of 1 indicates a coagulation level equivalent to a patient without VKA treatment. Values higher than this translate as a longer time in the formation of the blood clot and consequently more bleeding. The objective value of the INR will depend on the indication for which the drug was prescribed and can range between 2.5-3.5. VKA therapy must be adjusted by the attending physician to achieve the desired INR (Woolcombe et al., 2022).
Anti-factor Xa assay. It can be done by fluorescence or chromogenic. It measures the activity of activated coagulation factor X. Normal values correspond to 0.5-1.2UI/ml (Babin et al., 2017).
Within the qualitative studies, we found
Bleeding time: Used to assess platelet functionality, the most widely used is the Ivy technique, whose normal value is 8 to 10 minutes (González Guerrero & Montoro Ronsano, 2015).
Types of Oral Anticoagulants
Three types of oral anticoagulants are currently described: vitamin K-dependent (VKA), directacting anticoagulants (DOAC), and heparins.
Vitamin K (VKA) or coumarin antagonists
Among these types of drugs are Warfarin, Acenocoumarol, and Fenprocoumaron, all derivatives of hydroxycoumarin, with their pharmacokinetic and pharmacodynamic differences (Table 2).
Table 2 Pharmacokinetic and pharmacodynamic differences between warfarin, acenocoumarol, and fenprocoumaron (adapted from (Ufer, 2005).
| ANTICOAGULANT | VOLUME OF DISTRIBUTION (L/KG) | PROTEIN BINDING | PLASMA CONCENTRATION (UMOL/L) | ELIMINATION KINETICS |
| WARFARIN | 0,08-0,13 | >99% | 15-8 | First order |
| ACENOCUMAROL | 0,22-0,52 | >98% | 0.03-0.3 | Two-phase |
| FENPROCUMARON | 0,11-0,14 | >99% | 1,5-15 | First order |
They inhibit the carboxylation of vitamin K-dependent coagulation factors: Factor II (prothrombin), VII, IX, and X, in addition to inhibiting protein C and S.
They are indicated for the prevention and treatment of thrombosis and other types of cardiovascular conditions (atrial fibrillation, venous thromboembolism, and prosthetic heart valve) (Conway et al., 2017).
Monitoring
VKA levels are monitored by the INR. Which must be kept within the therapeutic range determined by the treating physician (Exposito, 2010). A subtherapeutic effect offers little protection against thromboembolic events, and an INR (>4) increases the risk of bleeding. The variability in the response between different individuals
and its wide range of interactions with other drugs and foods require frequent laboratory monitoring (Conway et al., 2017).
Interactions
Warfarin is highly bound to plasma proteins, so other substances or drugs that compete for the binding site displace Warfarin, enhancing the therapeutic effect of VKAs (Crader et al., 2022).
The inhibition of the expression/activity of the CYP450 enzymes (CYP2C9 for the S-enantiomer and CYP1A2, CYP2C19, CYP3A4 for the R-enantiomer of Warfarin), compromised in its metabolism also affect the effect of VKAs, therefore, plasma concentrations of VKAs can be altered when consumed together with drugs that modify the expression or availability of CYP450. VKAs can be altered when consumed together with drugs that modify the expression or availability of CYP450, such as some antimicrobial, antihypertensive, and antiinflammatory drugs, among others (Crader et al., 2022; Little et al., 2017) (Table 2) (Table 2).
Among the adverse effects that patients under treatment with VKA may present, we find hemorrhagic complications, teratogenic effects causing chondrodysplasia and nasal hypoplasia, skin necrosis, allergic reactions, liver damage, nephropathy, and alopecia (Patel et al., 2022)...
Table 3 Drug interactions with vitamin K antagonists. *VKA: vitamin K antagonist.
| Drug group | Increased AVK effect | Decreased AVK effect |
| Antimicrobials | Amoxicillin with Clavulanic acid, Azithromycin, Clarithromycin, Levofloxacin, Ritonavir, Tetracycline, Ciprofloxacin, Erythromycin, Fluconazole, Isoniazid, Miconazole | Riseofulvin, Nafcillin, Ribavirin, Rifampin, Dicloxacillin, Ritonavir |
| Cardiovascular | Amiodarone, Diltiazem, Fenofibrate, Clofibrate, Propafenone, Propranolol, Sulfinpyrazone, Aspirin | Cholestyramine, Bosentan, Spironolactone |
| Anti-inflammatories and immunomodulators | Phenylbutazone, Interferon, Aspirin, Paracetamol, Tramadol | Mesalazine, Azathioprine |
| Central nervous system | Alcohol (in case of coexisting liver disease), Citalopram, Entacapone, Sertraline, Disulfiram, Chloral hydrate, Fluvoxamine, Phenytoin, Tricyclic antidepressants (Amitriptyline, Clomipramine), Benzodiazepines | Barbiturates, Carbamazepine, Chlordiazepoxide. |
| Other drugs | Anabolic steroids, Zileuton, Zafirlukast, Fluorouracil, gemcitabine, Levamisole with Fluorouracil, Paclitaxel, Tamoxifen, Tolterodine, Thiamazole, L-Tyroxine, L-Tyroxine, Tamoxifen, Tamoxifen, Tamoxifen with Fluorouracil. | Mercaptopurine, Raloxifene, Multivitamin supplements, flu vaccination, chelating substances. |
Direct-acting anticoagulants (DOAC)
These types of blood thinners have gained popularity in recent years. DOACs are classified as direct inhibitors of thrombin (activated factor II) (Dabigatran) or direct inhibitors of activated factor X (Rivaroxaban, Apixaban and Edoxaban).
They are rapidly active drugs whose main indications are the prevention of acute myocardial infarction in non-valvular atrial fibrillation, thrombrophylaxis in knee or hip replacement surgery, and the treatment/ prevention of venous thromboembolism (Adcock & Gosselin, 2015; Conway et al., 2017).
Monitoring.
Unlike VKAs, they do not require periodic monitoring due to their pharmacokinetic and pharmacodynamic predictability.
This is advantageous for patient convenience and satisfaction. Despite this, there are various situations in which the clinician would want to address the precise effect of the anticoagulant to define treatment options, such as an emergency (e.g., trauma, hemorrhage, kidney or liver failure, among others) (Conway et al., 2017). Tests such as aPTT, PT, and TT have low sensitivity and specificity for this type of drug and lack an optimal dose response for monitoring DOACs.
For patients taking this type of anticoagulant, the results of the mentioned tests must be interpreted qualitatively to confirm the anticoagulant effect (Blann & Lip, 2014). Regarding the INR, its use cannot be recommended to monitor the use of DOACs, since the international sensitivity index was created as a thromboplastin correction factor specifically for VKAs (Winter et al., 2017).
Interactions.
The pharmacokinetics of Factor Xa inhibitors may be affected by inducers/inhibitors of CYP3A4 and/or p-glycoprotein 1. Regarding Dabigatran, inhibitors/inducers of p-glycoprotein 1 should not be indicated, as it is a specific substrate of this molecule (Di Minno et al., 2017).
They report fewer adverse reactions than VKAs (including intracerebral hemorrhage). Gastrointestinal disturbances have been reported with the use of Dabigatran, so if possible it should be replaced by another type of DOAC.
3. Heparins
Heparins are highly sulfated polysaccharides used as major anticoagulants. Indicated primarily as a component in extracorporeal therapy to maintain kidney blood flow during dialysis and cardiopulmonary oxygenation (Heparin, 2006).
Second, it has been used to prevent/treat deep vein thrombosis, pulmonary embolism, and ischemic complications of unstable angina, among others. It is administered intravenously or subcutaneously. Its activity is due to the ability to inhibit multiple factors in the coagulation cascade.
It binds to antithrombin as a serum protease inhibitor and targets coagulation factors such as Xa and IIa.
To optimize its anticoagulant activity and minimize the risk of bleeding, Heparin variants have been synthesized by fractionation (Beurskens et al., 2020).
The use of low molecular weight Heparin offers different advantages over unfractionated Heparin, such as greater bioavailability allowing more predictable dosing and lower prevalence of adverse effects. Thus, low molecular weight heparin has become the treatment of choice in clinical situations such as venous thromboembolism, major surgery, and acute coronary syndrome.
Unfractionated Heparin remains indicated in the prevention of coagulation with extracorporeal devices and patients with renal failure (Harenberg, 2011a, 2011b; Walenga et al., 2011).
Monitoring
The chromogenic anti-factor Xa assay is considered the gold standard to investigate the activity of low molecular weight heparins. Regarding aPTT, it has been reported that the prolongation of time in this assay depends on the reagent used.
Even so, this test is not useful in its monitoring, since this type of Heparin owes its effect mainly to factor Xa inhibition, while aPTT prolongation is dependent on thrombin activity (Babin et al., 2017; Despas et al., 2016).
Adverse effects. It is reported that due to its anticoagulant nature, bleeding or hemorrhages are to be expected. Bleeding sites include the adrenal gland, ovaries, and retroperitoneal area. This complication can occur virtually anywhere (Despas et al., 2016).
Heparin-induced thrombocytopenia is a serious antibody-associated reaction that results in abnormal and irreversible platelet aggregation, leading to life-threatening thromboembolic events (Harenberg, 2011b).
It is said that the long use of heparin can cause Osteoporosis and, consequently, increase the risk of fracture due to the inhibition of osteoblastic differentiation and its function (Heparin, 2006).
Bridging/Overlap Therapy
Bridging consists of substituting a longacting anticoagulant (usually warfarin) with a short-acting one (usually low molecular weight heparin) to limit the time of subtherapeutic anticoagulation levels and minimize thromboembolic risk. Although there is evidence that its use is limited, it continues to be used on a case-by-case basis (Nazar J. et al., 2018; Polania Gutièrrez & Rocuts, 2022).
Complications
The complications presented by patients under TACO treatment can be classified as hemorrhagic, which can be minor hemorrhage, major hemorrhage, and hemorrhage that compromises life; and non-hemorrhagic, such as skin necrosis, peripheral emboli, alopecia, and osteoporosis (Blann & Lip, 2014).
Dental management
Anamnesis. The patient’s clinical record must be completed carefully, informing the reason why the anticoagulant was prescribed, and past events of prolonged bleeding both in the dental office and in another context. It is important to verify all the necessary information before performing any invasive procedure so that the clinician has support (Iwabuchi et al., 2014).
Assessment of bleeding risk. Dental interventions can be divided into those in which bleeding is unlikely and others in which it is probable (Table 3). Based on this assessment, it is possible to determine which procedures can or cannot be performed in the dental office without major complications.
Table 4 Assessment of the risk of bleeding in dental procedures according to the “Scottish Dental Clinical Effectiveness Programme”.
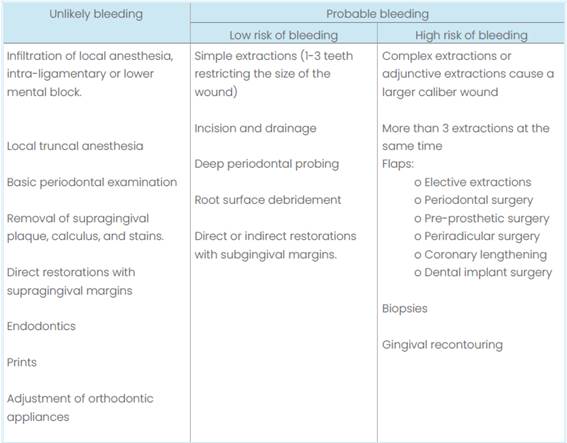
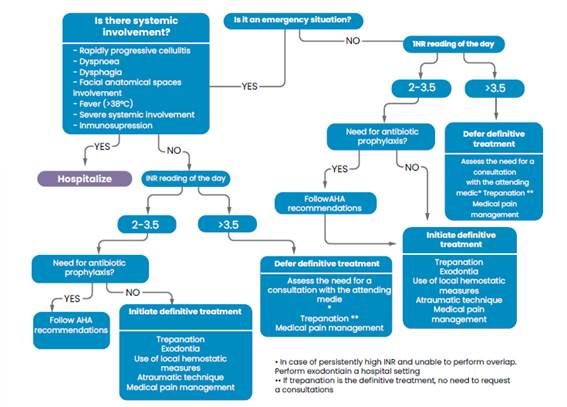
In patients with VKA, INR should be requested to determine whether or not it is safe to perform the corresponding intervention. This must be requested at least 24 hours before the procedure, but in patients with a stable INR, it is possible to accept an INR of no more than 72 hours (Woolcombe et al., 2022).
Continuing or stopping anticoagulant therapy in this type of patient is a decision of the treating physician and not of the dentist. In general, the literature reports that with INR values up to 4, invasive treatments can be performed (always having at hand all possible bleeding control measures).
If the INR value does not allow the procedure to be performed, trephination may be an option while the patient’s examinations are regularized, since it is considered a procedure where bleeding is unlikely to occur (Calcia et al., 2021; Winter et al., 2017). The flow of care in these patients is described in Figure 2.
For those patients taking DOAC, it is not necessary to suspend the medication, and the procedure can be carried out with the relevant hemostatic measures (Caliskan et al., 2017).
In the case of patients with injectable anticoagulants such as low molecular weight heparin, it should be treated without interrupting the medication. It is assumed that the risk of bleeding in these patients is dose-dependent. In patients using this drug with a prophylactic dose, the risk is lower than if they were taking a VKA (Andras et al., 2017).
Antibiotic prophylaxis. The use of preoperative antibiotics has been described as a predictor of postoperative bleeding, even though the mechanism of action is not well described.
Similarly, the use of postoperative antibiotics has been reported as a risk factor for intraoral bleeding.
A single antibiotic dose does not significantly affect the PT-INR of the anticoagulated patient, but its administration should be monitored in patients with an INR 3 for an increase in the therapeutic range (Huang et al., 2022; Yamada et al., 2020). Antibiotic prophylaxis, if necessary, should follow the recommendations of the American Heart Association (Wilson et al., 2021).
Anesthesia Adrenaline causes local constriction of blood vessels at the surgical site from 20 minutes to 2 hours. The occurrence of postoperative bleeding is affected by adrenaline at the time of bleeding control in the dental chair and 2-3 hours after the procedure (Inokoshi et al., 2021). Inferior nerve block has been reported to be a risk factor for postoperative bleeding (Huang et al., 2022). In general, it is safe to use local anesthetics in anticoagulated patients, as this procedure is classified as having a low risk of bleeding (Table 3).
Surgical procedure. An atraumatic surgical technique should be prioritized, avoiding as much as possible extending the wound (performing only one extraction or limiting root debridement to three teeth), and dividing the visits in which treatment is performed (Woolcombe et al., 2022). In general, the evidence reports that immediate and postoperative bleeding in simple extractions with an INR less than 3.0 is low (2%-3%) (Bajkin et al., 2014).
hemostasis. The use and selection of local hemostasis measures will depend largely on the degree of complexity of the intervention to be performed.
Measures reported in the literature include the use of sutures, alveolar fillers, and antifibrinolytic agents to control bleeding (Iwabuchi et al., 2014).
Sutures and alveolar fillings such as oxidized cellulose or gelite have been widely recognized as post-extraction hemostatic measures. Even so, not all guidelines necessarily recommend the use of sutures over these alveolar fillers because they can cause more damage to the perialveolar tissue (Iwabuchi et al., 2014).
Among the antifibrinolytics described in randomized clinical trials are tranexamic acid (ATX) in tablets, rinse and intravenous, and aminocaproic acid epsilon AACE) in tablets or intravenous. These agents bind irreversibly to plasminogen and block its interaction with fibrin.
The most studied to date is local TXA, whose efficacy is limited to preventing oral bleeding in anticoagulated patients (Engelen et al., 2018). Its availability as a rinse is not available in all countries, so injectable solutions are diluted for intraoral use (de Vasconcellos et al., 2017).
Regarding AACE, it is described as having antifibrinolytic potential in oral surgery, but to date, there are no randomized clinical trials that compare its effect with ATX (da Silva et al., 2018). The literature reports that DOACs have a lower incidence of postoperative bleeding than VKAs (Manfredini et al., 2021).
Pain management. Anticoagulants can interact with non-steroidal anti-inflammatory drugs (NSAIDs). The patient should be recommended to take Acetaminophen (if it is not contraindicated) over drugs such as Aspirin, Ibuprofen, Diclofenac, or Naproxen, since the latter increases the risk of bleeding. If the use of an NSAID is considered, it should be recommended for a minimum time and with gastric protection (Kent et al., 2018).
Control. The patient should be told that in the event of noticing a bleeding event between 24 hours and 7 days after the intervention, they should contact the center where the intervention was performed by telephone or go to the center in question or another facility that can control the bleeding (Inokoshi et al., 2021; Rocha et al., 2019).
Postoperative bleeding event. Defined as marked bleeding after mechanical compression with gauze for 30 minutes. These types of events should be monitored from the day of the procedure until one week after the intervention.
Conclusion
There are multiple protocols for the care of the anticoagulated patient who will undergo a minor oral surgery procedure. It is important to consider the anticoagulant used, the reason, its control, the procedure to be performed on the patient, and both intraoperative and postoperative hemostatic measures to be performed. After analyzing the above, it is noted that reducing the intake of the drug to perform the procedure can be more harmful to the patient and to the clinician, therefore it is suggested to maintain antithrombotic treatment and perform correct medical/surgical management.
Referencias
Adcock, D. M., & Gosselin, R. (2015). Direct Oral Anticoagulants (DOACs) in the Laboratory: 2015 Review. Thrombosis Research, 136 (1), 7-12. https://doi.org/10.1016/j.thromres.2015.05.001 [ Links ]
Andras, A., Sala Tenna, A., & Stewart, M. (2017). Vitamin K antagonists versus low-molecular-weight heparin for the long term treatment of symptomatic venous thromboembolism. Cochrane Database Syst Rev, 7(7), Cd002001. https://doi.org/10.1002/14651858.CD002001.pub3 [ Links ]
Babin, J. L., Traylor, K. L., & Witt, D. M. (2017). Laboratory Monitoring of Low-Molecular-Weight Heparin and Fondaparinux. Semin Thromb Hemost, 43(3), 261-269. https://doi.org/10.1055/s-0036-1581129 [ Links ]
Bajkin, B. V., Selaković, S. D., Mirković, S. M., Šarčev, I. N., Tadič, A. J., & Milekič, B. R. (2014). Comparison of efficacy of local hemostatic modalities in anticoagulated patients undergoing tooth extractions. Vojnosanit Pregl, 71(12), 1097-1101. https://doi.org/10.2298/vsp1412097b [ Links ]
Beurskens, D. M. H., Huckriede, J. P., Schrijver, R., Hemker, H. C., Reutelingsperger, C. P., & Nicolaes, G. A. F. (2020). The Anticoagulant and Nonanticoagulant Properties of Heparin. Thromb Haemost, 120 (10), 1371-1383. https://doi.org/10.1055/s-0040-1715460 [ Links ]
Blann, A. D., & Lip, G. Y. (2014). Laboratory monitoring of the non-vitamin K oral anticoagulants. J Am Coll Cardiol, 64(11), 1140-1142. https://doi.org/10.1016/j.jacc.2014.07.010 [ Links ]
Calcia, T. B. B., Oballe, H. J. R., de Oliveira Silva, A. M., Friedrich, S. A., & Muniz, F. (2021). Is alteration in single drug anticoagulant/antiplatelet regimen necessary in patients who need minor oral surgery? A systematic review with meta-analysis. Clin Oral Investig, 25 (6), 3369-3381. https://doi.org/10.1007/s00784-021-03882-z [ Links ]
Caliskan, M., Tükel, H. C., Benlidayi, M. E., & Deniz, A. (2017). Is it necessary to alter anticoagulation therapy for tooth extraction in patients taking direct oral anticoagulants? Med Oral Patol Oral Cir Bucal, 22(6), e767-e773. https://doi.org/10.4317/medoral.21942 [ Links ]
Conway, S. E., Hwang, A. Y., Ponte, C. D., & Gums, J. G. (2017). Laboratory and Clinical Monitoring of Direct Acting Oral Anticoagulants: What Clinicians Need to Know. Pharmacotherapy, 37(2), 236-248. https://doi.org/10.1002/phar.1884 [ Links ]
Costa-Tort, J., Schiavo-Di Flaviano, V., González Navarro, B., Jané-Salas, E., Estrugo-Devesa, A., & López- López, J. (2021). Update on the management of anticoagulated and antiaggregated patients in dental practice: Literature review. Journal of clinical and experimental dentistry, 13(9), e948-e956. https://doi.org/10.4317/jced.58586 [ Links ]
Crader, M. F., Johns, T., & Arnold, J. K. (2022). Warfarin Drug Interactions. In StatPearls. StatPearls Publishing Copyright 2022, StatPearls Publishing LLC. [ Links ]
da Silva, R. V., Gadelha, T. B., Luiz, R. R., & Torres, S. R. (2018). Intra-alveolar epsilon-aminocaproic acid for the control of post-extraction bleeding in anticoagulated patients: randomized clinical trial. International Journal of Oral and Maxillofacial Surgery, 47(9), 1138-1144. https://doi.org/10.1016/j.ijom.2018.02.013 [ Links ]
de Vasconcellos, S. J., de Santana Santos, T., Reinheimer, D. M., Faria, E. S. A. L., de Melo, M. F., & Martins-Filho, P. R. (2017). Topical application of tranexamic acid in anticoagulated patients undergoing minor oral surgery: A systematic review and meta-analysis of randomized clinical trials. J Craniomaxillofac Surg, 45(1), 20-26. https://doi.org/10.1016/j.jcms.2016.10.001 [ Links ]
Despas, N., Larock, A. S., Jacqmin, H., Douxfils, J., Chatelain, B., Chatelain, M., & Mullier, F. (2016). Heparin monitoring: clinical outcome and practical approach. Ann Biol Clin (Paris), 74(6), 637-652. https://doi.org/10.1684/abc.2016.1198 (Suivi biologique de l’héparinothérapie : intérêt clinique et aspects pratiques.) [ Links ]
Di Carlo, A., Bellino, L., Consoli, D., Mori, F., Zaninelli, A., Baldereschi, M., Cattarinussi, A., D’Alfonso, M. G., Gradia, C., Sgherzi, B., Pracucci, G., Piccardi, B., Polizzi, B., & Inzitari, D. (2019). Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European Union: the FAI Project. Europace, 21(10), 1468-1475. https://doi.org/10.1093/europace/euz141 [ Links ]
Di Minno, A., Frigerio, B., Spadarella, G., Ravani, A., Sansaro, D., Amato, M., Kitzmiller, J. P., Pepi, M., Tremoli, E., & Baldassarre, D. (2017). Old and new oral anticoagulants: Food, herbal medicines and drug interactions. Blood Reviews, 31 (4), 193-203. https://doi.org/https://doi.org/10.1016/j.blre.2017.02.001 [ Links ]
Engelen, E. T., Schutgens, R. E. G., Mauser‐Bunschoten, E. P., van Es, R. J. J., & van Galen, K. P. M. (2018). Antifibrinolytic therapy for preventing oral bleeding in people on anticoagulants undergoing minor oral surgery or dental extractions. Cochrane Database of Systematic Reviews (7). https://doi.org/10.1002/14651858.CD012293.pub2 [ Links ]
Expósito, M. C. (2010). Tratamiento con anticoagulantes orales: inicio, ajuste y precauciones en su utilización. Avances en Diabetología, 26(1), 17-20. https://doi.org/https://doi.org/10.1016/S1134-3230(10)61004-6 [ Links ]
Gale, A. J. (2011). Continuing education course #2: current understanding of hemostasis. Toxicol Pathol, 39(1), 273-280. https://doi.org/10.1177/0192623310389474 [ Links ]
Gazit, Y., Jacob, G., & Grahame, R. (2016). Ehlers-Danlos Syndrome-Hypermobility Type: A Much Neglected Multisystemic Disorder. Rambam Maimonides Med J, 7(4). https://doi.org/10.5041/rmmj.10261 [ Links ]
Gomez, K., Anderson, J., Baker, P., Biss, T., Jennings, I., Lowe, G., & Platton, S. (2021). Clinical and laboratory diagnosis of heritable platelet disorders in adults and children: a British Society for Haematology Guideline. Br J Haematol, 195 (1), 46-72. https://doi.org/10.1111/bjh.17690 [ Links ]
González Guerrero, C., & Montoro Ronsano, J. B. (2015). Physiopathology and treatment of critical bleeding: a literature review. Farmacia Hospitalaria, 39, 382-398. http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1130-63432015000600008&nrm=iso [ Links ]
Grymonprez, M., Simoens, C., Steurbaut, S., De Backer, T. L., & Lahousse, L. (2022). Worldwide trends in oral anticoagulant use in patients with atrial fibrillation from 2010 to 2018: a systematic review and meta-analysis. Europace, 24(6), 887-898. https://doi.org/10.1093/europace/euab303 [ Links ]
Guerrero, B., & López, M. (2015). Generalidades del sistema de la coagulación y pruebas para su estudio. Investigación Clínica, 56, 432-454. http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0535-51332015000400010&nrm=iso [ Links ]
Harenberg, J. (2011a). Differences of present recommendations and guidelines for generic low-molecularweight heparins: is there room for harmonization. Clin Appl Thromb Hemost, 17(6), E158-164. https://doi.org/10.1177/1076029610392216 [ Links ]
Harenberg, J. (2011b). Overview on guidelines and recommendations for generic low-molecular-weight heparins. Thromb Res, 127 Suppl 3, S100-104. https://doi.org/10.1016/s0049-3848(11)70027-5 [ Links ]
Hatton, C. S. R., Hughes-Jones, N. C., & Hay, D. (2013). Hematología: Diagnóstico y tratamiento. Editorial El Manual Moderno. https://books.google.cl/books?id=xH7-CAAAQBAJ [ Links ]
Heparin. (2006). In Drugs and Lactation Database (LactMed). National Institute of Child Health and Human Development. [ Links ]
Huang, J., Liu, J., Shi, H., Wu, J., Liu, J., & Pan, J. (2022). Risk factors for bleeding after dental extractions in patients receiving antithrombotic drugs - A case control study. Journal of Dental Sciences, 17(2), 780-786. https://doi.org/https://doi.org/10.1016/j.jds.2021.10.005 [ Links ]
Inokoshi, M., Kubota, K., Yamaga, E., Ueda, K., & Minakuchi, S. (2021). Postoperative bleeding after dental extraction among elderly patients under anticoagulant therapy. Clinical Oral Investigations, 25(4), 2363-2371. https://doi.org/10.1007/s00784-020-03559-z [ Links ]
Iwabuchi, H., Imai, Y., Asanami, S., Shirakawa, M., Yamane, G.-y., Ogiuchi, H., Kurashina, K., Miyata, M., Nakao, H., & Imai, H. (2014). Evaluation of postextraction bleeding incidence to compare patients receiving and not receiving warfarin therapy: a cross-sectional, multicentre, observational study. BMJ Open, 4(12), e005777. https://doi.org/10.1136/bmjopen-2014-005777 [ Links ]
Kent, A. P., Brueckmann, M., Fraessdorf, M., Connolly, S. J., Yusuf, S., Eikelboom, J. W., Oldgren, J., Reilly, P. A., Wallentin, L., & Ezekowitz, M. D. (2018). Concomitant Oral Anticoagulant and Nonsteroidal Anti-Inflammatory Drug Therapy in Patients With Atrial Fibrillation. J Am Coll Cardiol, 72(3), 255-267. https://doi.org/10.1016/j.jacc.2018.04.063 [ Links ]
Kornej, J., Börschel, C. S., Benjamin, E. J., & Schnabel, R. B. (2020). Epidemiology of Atrial Fibrillation in the 21st Century. Circulation Research, 127(1), 4-20. https://doi.org/doi:10.1161/CIRCRESAHA.120.316340 [ Links ]
Kumar, V., Abbas, A. K., & Aster, J. C. (2015). Robbins y Cotran. Patología estructural y funcional. Elsevier Health Sciences Spain. https://books.google.cl/books?id=fOJiCAAAQBAJ [ Links ]
LaPelusa, A., & Dave, H. D. (2022). Physiology, Hemostasis. In StatPearls. StatPearls Publishing Copyright 2022, StatPearls Publishing LLC. [ Links ]
Little, J. W., Miller, C., Miller, C. S., & Rhodus, N. L. (2017). Little and Falace’s Dental Management of the Medically Compromised Patient. Elsevier - Health Sciences Division. https://books.google.cl/books?id=8p-RAQAACAAJ [ Links ]
Manfredini, M., Poli, P. P., Creminelli, L., Porro, A., Maiorana, C., & Beretta, M. (2021). Comparative Risk of Bleeding of Anticoagulant Therapy with Vitamin K Antagonists (VKAs) and with Non-Vitamin K Antagonists in Patients Undergoing Dental Surgery. Journal of Clinical Medicine, 10(23), 5526. https://www.mdpi.com/2077-0383/10/23/5526 [ Links ]
Nazar J., C., Cárdenas C., A., Coloma D., R., Contreras C., J. I., Molina, I., Miranda H., P., & Fuentes H., R. (2018). Manejo perioperatorio de pacientes con tratamiento anticoagulante crónico. Revista chilena de cirugía, 70, 84-91. http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0718-40262018000100084&nrm=iso [ Links ]
Onishi, A., St Ange, K., Dordick, J. S., & Linhardt, R. J. (2016). Heparin and anticoagulation. Front Biosci (Landmark Ed), 21(7), 1372-1392. https://doi.org/10.2741/4462 [ Links ]
Patel, S., Singh, R., Preuss, C. V., & Patel, N. (2022). Warfarin. In StatPearls. StatPearls Publishing Copyright 2022, StatPearls Publishing LLC. [ Links ]
Polania Gutierrez, J. J., & Rocuts, K. R. (2022). Perioperative Anticoagulation Management. StatPearls Publishing, Treasure Island (FL). http://europepmc.org/abstract/MED/32491522 [ Links ]
Rocha, A. L., Oliveira, S. R., Souza, A. F., Travassos, D. V., Abreu, L. G., Ribeiro, D. D., & Silva, T. A. (2019). Bleeding assessment in oral surgery: A cohort study comparing individuals on anticoagulant therapy and a nonanticoagulated group. Journal of Cranio-Maxillofacial Surgery, 47(5), 798-804. https://doi.org/https://doi.org/10.1016/j.jcms.2019.01.049 [ Links ]
Smith, S. A., Travers, R. J., & Morrissey, J. H. (2015). How it all starts: Initiation of the clotting cascade. Crit Rev Biochem Mol Biol, 50(4), 326-336. https://doi.org/10.3109/10409238.2015.1050550 [ Links ]
Ufer, M. (2005). Comparative pharmacokinetics of vitamin K antagonists: warfarin, phenprocoumon and acenocoumarol. Clin Pharmacokinet, 44 (12), 1227-1246. https://doi.org/10.2165/00003088-200544120-00003 [ Links ]
Walenga, J. M., Jackson, C. M., & Kessler, C. M. (2011). Low molecular weight heparins differ substantially: impact on developing biosimilar drugs. Semin Thromb Hemost, 37(3), 322-327. https://doi.org/10.1055/s-0031-1274515 [ Links ]
Wilson, W. R., Gewitz, M., Lockhart, P. B., Bolger, A. F., DeSimone, D. C., Kazi, D. S., Couper, D. J., Beaton, A., Kilmartin, C., Miro, J. M., Sable, C., Jackson, M. A., & Baddour, L. M. (2021). Prevention of Viridans Group Streptococcal Infective Endocarditis: A Scientific Statement From the American Heart Association. Circulation, 143 (20), e963-e978. https://doi.org/10.1161/cir.0000000000000969 [ Links ]
Winter, W. E., Flax, S. D., & Harris, N. S. (2017). Coagulation Testing in the Core Laboratory. Laboratory Medicine, 48(4), 295-313. https://doi.org/10.1093/labmed/lmx050 [ Links ]
Woolcombe, S. A., Ball, R. E., & Patel, J. P. (2022). Managing direct oral anticoagulants in accordance with the Scottish Dental Clinical Effectiveness Programme guidance for patients undergoing dentoalveolar surgery. British Dental Journal, 232(8), 547-554. https://doi.org/10.1038/s41415-022-3999-y [ Links ]
Yamada, S.-i., Hasegawa, T., Soutome, S., Yoshimura, H., Miyakoshi, M., Ueda, N., Okamoto, K., Hishida, S., Rokutanda, S., Nakahara, H., Fujita, S., Akashi, M., Kitagawa, Y., Kirita, T., Shibuya, Y., Umeda, M., & Kurita, H. (2020). Prevalence of and risk factors for postoperative hemorrhage after lower third molar extraction on warfarin therapy: a multicenter retrospective study in Japan. Odontology, 108(3), 462-469 https://doi.org/10.1007/s10266-019-00474-y [ Links ]











 texto em
texto em