Revista de Biología Tropical
versão On-line ISSN 0034-7744versão impressa ISSN 0034-7744
Rev. biol. trop vol.51 no.1 San José Mar. 2003
Abstract
Phenotypic sexual dimorphism seems to be rare in the Ramphastidae family, except in Pteroglossus viridis and in the genus Selenidera. Many breeders of wild birds believe that specimens of Ramphastos toco can be sexed using bill characteristics. In this study, various discriminant phenotypic variables were analyzed in birds which were sexed cytogenetically. Fifty-one specimens of R. toco and 20 R. dicolorus were studied. The statistically significant parameters which served to distinguish the sex in these species were the length of the culmen and tomium, length of the lower corneous beak and the cloaca. Using these parameters, capitive bird breeders can determine sex of R. toco specimens by phenotypic analysis and form breeding couples more quickly.
Key words: sexual dimorphism, Ramphastos, Ramphastidae.
The family Ramphastidae, order Piciformes, includes species found in neotropical forests from Mexico to Peru, Brazil and the northeast of Argentina (Sick 1997). Normally, Ramphastidae show no phenotypic differences between the sexes, except P. viridis and the genus Selenidera, in which the sexes are differentiated by their beak and breast colour. Adult R. toco males are heavier than females whereas females may have longer beaks than males (Sick 1997).
Breeders of exotic bird generally consider that the beak of male R. toco is bigger than that of females and this parameter is frequently used to identify male specimens in their collections. Although there is evidence that beak characteristics may serve to indicate the sex of Ramphastidae species, no study has yet examined the usefulness of this parameter compared with more conventional methods of sexing.
Chromosomal analysis (Omura 1976, Harris and Walters 1982, Sasaki et al. 1983, 1984, Lucca and Rocha 1992, Rocha et al. 1995) and genetic molecular techniques (Lessells and Mateman 1996, Miyaki et al. 1995) allow unmistakable identification of the sex in birds and they have been widely used to sex birds.
We examined the correlation between phenotypic characteristics of R. toco and R. dicolorus and sex in specimens in which the sex was determined by cytogenetic analysis. Fifty one specimens of R. toco (25 males and 26 females) and 20 specimens of R. dicolorus (9 males and 11 females) were analyzed. All specimens were mature (adult) individuals. Dermal pulp tissue obtained from growing feathers was used for chromosomal analysis according Rocha et al. (1995) (Figs. 1 and 2).
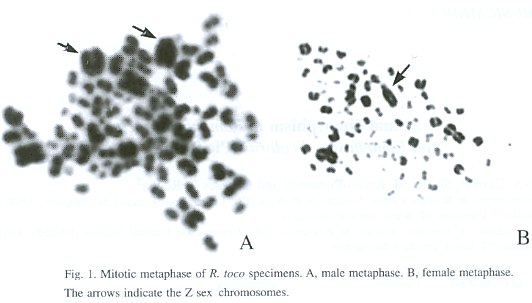
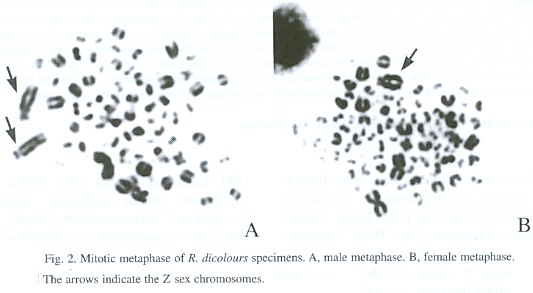
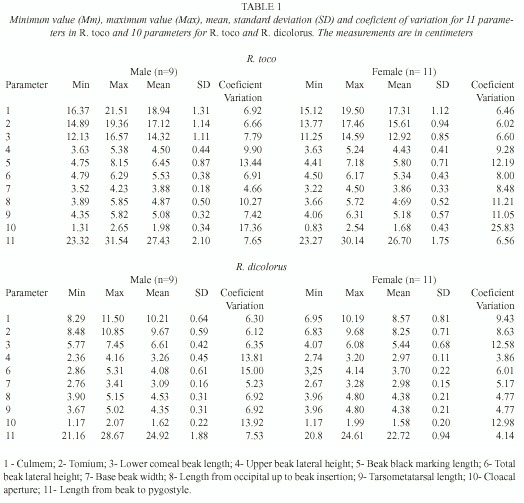
Eleven parameters were measured for R. toco and 10 for R. dicolorus. The measurements chosen were based on standard phenotypic ornithological parameters (Sick 1997). A steel pachymeter was used for making the measurements. The data obtained have a normal distribution and were subsequently analyzed by multivariate analysis.
Possible correlations between anatomical data and the sex of the specimens were assessed using a linear discriminate function (L.D.F.) (Morrison 1967) to calculate the function F (x) = Â(pi=1 ) ai x i, where p is the number of variables, ai is the variable coefficient and i represents each of the analyzed variables. The value of the function F(x) was calculated for males and females.
For R. toco and R. dicolorus, the sex-discriminating variables were the culmem (upper beak dorsal line), tomium (upper beak ventral line), lower corneous beak length (lower beak ventral line) (p<0.001) and cloacal aperture (distance between extremities of the pubic bone) (p<0.01), all of which were bigger in male than in females (Table 1). In R. dicolorus, the upper beak lateral height and total beak lateral height in males were greater than or similar to those of females (0.05<p<0.1) (Table 1). In neither of the species was there a significant correlation between the sex when other variables were measured, although the latter were still used to construct a discriminatory function.
Using analysis of variance and the variable coefficient for the values of L.D.F., a specimens sex could be obtained using the equations below, where x is the mean value for each of the parameters analyzed.
For R. toco,
F(x) = 0.71253.x1 + 3.48442.x 2 - 0.15033.x3 + 0.08571.x4 - 0.98125.x5 . + 1.40075x6 - 5.54331.x7 + 0.53348.x8 - 0.67981.x9 + 1.55211.x10 - 0.1128.x11
The mean value of F(x) was 23.44 for male and 19.49 for females. The mean F(x) for males and females combined was 21.47.
For R. dicolorus,
F(x) = 6.7795.x1 - 6.60021.x 2 + 5.08629.x3 - 10.68596.x4 + 3.4396.x6 - 15.35236.x7 + 7.67014.x8 + 0.1839.x9 + 0.512.x10 + 2. 823.x11
The mean value of F(x) was 75.74 for males and 65.91 for females. The mean F(x) for males and females combined was 71.73. Thus, for both species, an F(x) greater than the overall mean (21.47 for R. toco and 71.73 for R. dicolorus) indicated a male and whereas an F(x) lower than the overall mean indicated a female.
Length measurements and beak shapes are used by breeders to distinguish the sexes in R. toco. For this reason, we examined a larger number of specimens of this species. In addition, this species is common in zoological gardens and therefore easier to obtain. Höfling (1991) reported significant differences between the sexes following an analysis of the upper beak lateral height in 11 specimens of R. tucanus with females having significantly greater measurements than males.
Analyses of this same parameter in R. toco showed no significant differences between the sexes. For R. dicoloru s, this measurement in males was greater than or equal to that of females (0.05<p<0.10). This result was the opposite of that reported by Höfling (1991) for R. tucanus, even though the two species show a similar biotype.
Duarte and Barbosa (1992) examined three biometric parameters related to beak size in three species of Ramphastos and established an equation to determine phenotypic sex in each of the species. The error inherent in sexing R. toco, R. vitellinus and R. tucanus by this method was up to 30%, 19% and 14%, respectively. Since the measurements were obtained from museum specimens, errors in the sex and age (adult/young) recorded for the specimens may have influenced the accuracy of the method.
Applying the biometric values obtained in the present study to the equation proposed by Duarte and Barbosa (1992), only three of the 25 R. toco males were identified as males. This large error in sexing indicated the need to review the equation proposed by these authors, as well as the need to include additional measurements. Analysis of variance (ANOVA) of the variables selected for the two Ramphastos species indicated that only four were significant for sex discrimination. In both species, the measurements made were significantly greater in males than females.
There was a significant correlation among sex discriminant and non-discriminant variables in both species when the variables were combined in a two at a time. The lower corneous beak length (discriminating sex), showed a positive correlation with the nondiscriminant variable of beak marking length (p<0.01), base beak width (p<0.01), metatarsal length (p<0.05) and length from beak to pygostyle (p<0.01). This same parameter was also correlated with the discriminant variables of culmem length (p<0.01), lower corneous beak length (p<0.01) and cloacal aperture (p<0.05). Similarly, non-discriminant variables correlated with themselves. For example, base beak width and length from beak to pygostyle (p<0.01). Thus, to establish a sex discriminant function for each of the species, all of the variables analyzed were considered, each one being followed by its respective coefficient.
A significant difference between the sexes was observed for R. toco (p<0.05) using the proposed equation for all 11 variables. Of 22 specimens classified as males, only one was actually a female. Similarly, of 29 specimens classified as females, four were actually males. Thus, males were more likely to be confused with females (4 out of 29 or 13.8%) whereas females were rarely big enough to be classified as males (1 out of 22 or 4.5%). When the equation was applied to a given specimen and a male was indicated, the probability of being correct was 95.5%. For females, the probability of being correct was 86.2%.
The four sex discriminating parameters analyzed for R. dicolorus, revealed no significant differences between the sexes (p<0.05), perhaps because of the small sample size (20 specimens). However, the L.D.F. indicated different means for females (F(x)=65.91) and males (F(x)=77.54).
When the equation was applied to 20 specimens of known sex, 12 were classified as females, although one was actually a male. All of the specimens classified as males were indeed males. Thus, in this species it was possible to confuse small males with females (1 out of 12 or 8.0%). However, rarely were females big enough to be confused with males (none was found in the sample used).
For these two species of Ramphastos, the length of the lower corneous beak is an effective indicator of the birds sex. Other parameters may contribute to sex identification.
The results show that the sex of R. toco and R. dicolorus can be determined by pheno-typic analysis and for this it should be taken the measures of the phenotypic variables. This is an easy, quick and low cost method that could be used by breeders, since the only material required is a pachymeter used to determine the animals measurements.
Acknowledgments
We are greatly indebted to those Institutions in São Paulo State which allowed collection of growing feathers of the specimens used in this work. The authors also thank Dr. Paulo Roberto Curi (Scientific Adviser, FMVZ, UNESP) for the statistical analysis. This work was supported by CAPES.
Resumen
Con frecuencia, en la familia Ramphastidae no hay un dimorfismo sexual aparente, excepto en Pteroglossus viridis y en el género Selenidera. Muchos criadores de aves silvestres creen que los especímenes de Ramphastos toco pueden ser sexados usando las caracteríticas del pico. En este estudio, fueron analizadas algunas variables fenotípicas discriminantes en aves cuyo sexo fue previamente determinado con metodos citogenéticos. Un total de 51 especimenes de R. toco y 20 de R. dicolorus fueron estudiados. Los parámetros estadísticos significativos que son útiles para distinguir el sexo en estas especies son la longitud del culmen y del tomium, la longitud del pico corneo inferior y de la cloaca. Usando estos parámetros, los criadores de aves cautivas pueden sexar los especimenes de Ramphastos toco mediante análisis fenotípico y formar parejas reproductoras más rapidamente.
References
Duarte, J.M. B. & J.C. Barbosa. 1992. Sexagem do gênero Ramphastos por mensurações de bico, utilizandose análise discriminante. Anais do XIV Congresso da Sociedade de Zoológicos do Brasil, p.19.
Harris, T. & C. Walters. 1982. Chromosomal sexing of the black shouldered kite (Elanus caeruleus - Aves: Accipitridae). Genetica 60: 19-20. [ Links ]
Höfling, E. 1991. Étude comparative du crâne chez des Ramphastidae (Aves, Piciformes). Bonner Zoologische Beitraege 42: 55-65. [ Links ]
Lessells, K. & C. Mateman. 1996. Molecular sexing of birds. Nature 383: 761-762. [ Links ]
Lucca, E.J. & G.T. Rocha. 1992. Citogenética de aves. Bol. Mus. Para. Emílio Goeldi, Série Zool. 8: 33-68. [ Links ]
Miyaki, C.Y., J. M.B. Duarte, R. Caparroz, I. Biasia, A.L.V. Nunes & A. Wajntal. 1995. Identificação do sexo de psitacídeos pelo DNA. Braz. J. Genet. (suppl) 18(3): 315.
Morrison, D. F. 1967. Multivariate statistical methods. McGraw Hill, New York.
Omura, Y. 1976. Sex determination by chromosomes in seven species of birds. Jpn. J. Vet. Sci. 38: 281-288. [ Links ]
Rocha, G.T., M.S. Santos, R.C. Amaro & E.J. Lucca. 1995. Análise cromossômica e determinação do sexo de aves ameaçadas de extinção. Braz. J. Genet. (suppl) 18(3): 479. [ Links ]
Sasaki, M., N. Takagi & C. Nishida. 1983. Chromosomal diagnosis of sex in birds, its practice and application. J. Jpn. Assoc. Zool. Gardens & Aquariums 4: 105-113.
Sasaki, M., N. Takagi & C. Nishida. 1984. Current profiles of avian cytogenetics, with notes on chromosomal diagnosis of sex in birds. The Nucleus 27: 73-80. [ Links ]
Sick, H. 1997. Ornitologia Brasileira, uma introdução. Nova Fronteira, Rio de Janeiro, pp. 492-503. [ Links ]
1 Departamento de Biologia Celular, Instituto de Biologia (IB), Universidade Estadual de Campinas, (UNICAMP), 13084-971 Campinas, SP, Brasil; shirlei@unicamp.br
2 Departamento de Genética, Instituto de Biociências (IB), Universidade Estadual Paulista (UNESP), 18618-000 Botucatu, SP, Brasil; gtrocha@ibb.unesp.br












 uBio
uBio 

