Revista de Biología Tropical
versão On-line ISSN 0034-7744versão impressa ISSN 0034-7744
Rev. biol. trop vol.51 no.1 San José Mar. 2003
Abstract
This study provides the first semi-quantititative account of the benthic campanulariid hydroids from Northern Bahia (Brazil), down to a depth of 60 m, based largely on collections obtained since 1992. Colonies were collected from six habitats along the coast of Salvador City, Todos os Santos Bay, Itaparica Island and at the northernmost part of the coast of the State of Bahia. From the 982 colonies examined, nine species were recorded: Campanularia hincksii, Clytia gracilis, C. hemisphaerica, C. hummelincki, C. linearis, C. macrotheca, C. noliformis, Obelia bidentata and O. dichotoma. Following a defined abundance scale, Clytia gracilis and C. noliformis were the most abundant species, whereas Campanularia hincksii and Clytia hummelincki were rare. Cluster analysis of relative abundance data revealed sandy shores had a markedly different hydroid community from other habitats. A simplified identification key, redescriptions, illustrations and data on nematocyst compliment are provided for each species. Campanularia hincksii, Clytia macrotheca and C. noliformis are reported from Brazil for the first time.
Key Words: Campanularia, Clytia, Obelia, hydroid, cnidome, Brazil.
The Campanulariidae is a comparatively well-known hydroid family (Calder 1991) and most of its genera are nearly cosmopolitan, with records from all oceans (Cornelius 1982). Although significant progress has been made on this group, including accurate description of cnidome (Östman 1979a, 1979b, 1982a, 1987 and 1988) and biochemical taxonomy (Östman 1982b), many aspects of its biology and ecology remain unclear. This lack of knowledge is particularly evident in South America. In Brazil, research on benthic hydroids started in the 1940 (Vannucci Mendes 1946; Vannucci 1949, 1950). Some reports appeared three decades later (Narchi and Hebling 1975; Mayal 1973, 1983; Migotto 1993; Silveira and Migotto 1984, 1991, 1992; Migotto and Silveira 1987); however, except for the works of Mayal (1973, 1983), these studies concentrated mainly on species from the south coast of the country.
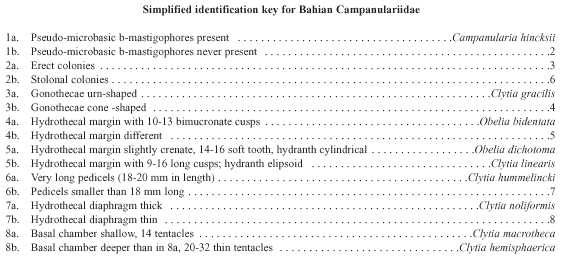
The State of Bahia (Northeastern Brazil) has a very extensive coastline (1 120 km) with many different ecosystems, including mangroves, rocky and sandy shores, beach rocks (bedrock appearing through an eroding sandy shore), coral-algal build ups and coral reefs. Although there are some hydroid descriptions from these environments, the general hydroid fauna is poorly known except for the taxonomic accounts of Kelmo and Peixinho (1996), Souza (1997) and Kelmo and Santa-Isabel (1998), but these did not concentrate on campanulariids. There is no complete systematic inventory of leptothecate hydroids. Many aspects of the biology and ecology of these animals remain unclear, discouraging research into the group. The purpose of this report is to provide the first semiquantitative account of the biodiversity of benthic campanulariid hydroids from Northern Bahia, down to a depth of 60 m, based largely on collections obtained since 1992. We present a simplified identification key, redescriptions and illustrations for all species.
Material and methods
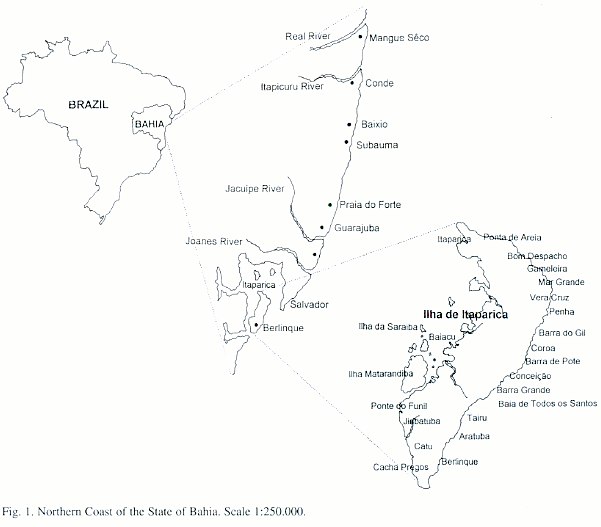
The present study considers material from the northernmost coast of the State of Bahia, Salvador City, Todos os Santos Bay and Itaparica Island (Fig. 1).
Hydroids were collected either along transect lines, during snorkelling and SCUBA, and/or using a Petersens dredge. Colonies were anaesthetised in 7.8% solution of MgCl 2 , fixed in 4% formaldehyde, preserved in 70% ethanol and stored at the Scientific Collection of Cnidarians of the Departamento de Zoologia do Instituto de Biologia da Universidade Federal da Bahia - Brazil (UFBA-CNI-HYD). In this paper, we refer the catalogue number [N] for each studied species. Colonies were examined under high-power stereoscopic and brightfield microscopy, and taxonomic identification was based on the literature cited in the text of this paper. All descriptions and illustrations provided herein are from the examined material.
The listed synonymy, following Calder (1991), Cornelius (1982, 1995) and Migotto (1993), was verified by examination of the original references. In most cases, only one significant record has been cited to document occurrences worldwide. Hydroid classification has been modified extensively by Petersen (1979, 1990), Werner (1984), Bouillon (1985) and Calder (1988, 1991, 1997); however, in the interests of simplicity, the classification adopted here follows Cornelius (1995).
Nematocysts were examined by compressing pieces of tissue, or entire individuals, between a slide and coverslip (Calder 1991). Specimens were treated with a 10% solution of sodium hypochlorite for 5-10 secs, and rinsed twice in distilled water prior to slide preparation (Kelmo and Santa-Isabel 1998). Nematocyst categories were identified according to the classification of Weill (1934) and Östman (1988). Length and width measurements were made from undischarged nematocysts using an ocular micrometer. For each species, at least 10 nematocysts of each type were measured for determining size ranges.
Relative abundance analysis was performed on hydroid samples from each ecosystem according to the progressive scale proposed by Peixinho and Peso-Aguiar (1989). Fourth-root transformed abundance data from each ecosystem were used to compute Bray-Curtis similarity coefficients (Bray and Curtis 1957) for each pair of ecosystem. The similarity matrix was subjected to cluster analysis using the program PRIMER (Plymouth Routines in Multivariate Ecological Resource, Carr 1996). Clustering utilised a hierarchical agglomerative method with group-average linking, resulting in a dendogram.
Results
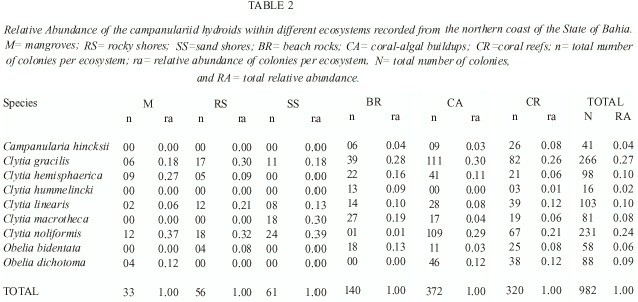
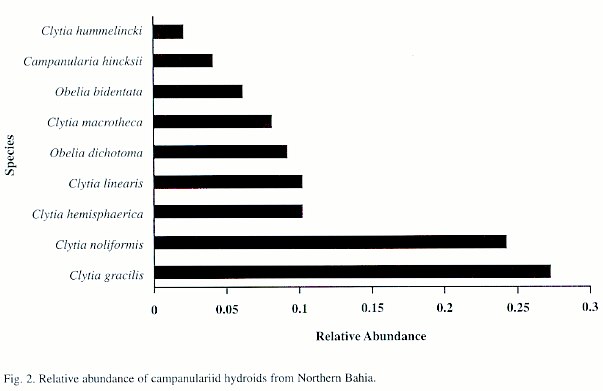
Of the 982 colonies examined, nine species were recorded: Campanularia hincksii, Clytia gracilis, C. hemisphaerica, C. hummelincki, C. linearis, C. macrotheca, C. noliformis, Obelia bidentata and O. dichotoma (Table 1). Clytia gracilis and C. noliformis were the most abundant species (Table 2), whereas Campanularia hincksii and Clytia hummelincki were rare (Fig. 2).
The Bray-Curtis similarity dendogram (Fig. 3) formed three distinct clusters at 63% similarity. The first cluster isolated sandy shore samples, the second group comprised man-grove and rocky shore fauna, and the third comprised all the samples from the northern coast of the State of Bahia (beach rocks, coral-algal build ups and coral reefs).

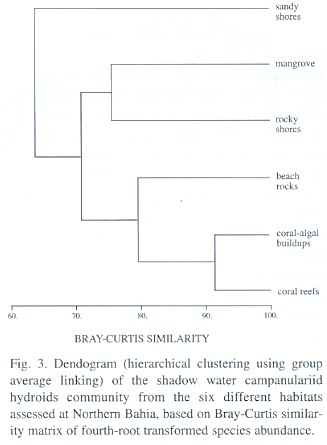
Systematic Account
Campanularia volubilis Hincks, 1853; Campanularia hincksii Alder, 1856; Calder, 1991; Cornelius, 1995; Campanularia (Orthopyxis) hincksii Verrill, 1873; Campanularia hincksii grandis Billard, 1906a; Campanularia (Eu-Campanularia) hincksii Broch, 1933; Campanularia emarginata Fraser, 1938a; Campanularia altitheca Fraser, 1948; Campanularia (Campanularia) hincksii Vervoort, 1968.
Material studied: Arembepe Beach, on external bank of beach rocks, 10-11 m depth, 6.iv.1996, six colonies, 5- 6.5 mm high, female gonophores observed [N 1752]; Northern coast of Bahia, on coral algal buildups, 20-25 m depth, 20.xii.1992, four colonies, 6-6.8 mm high, male gonophores [N 1755]; 30 m depth, 16.iii.1993, two colonies, 5.5 and 6.2 mm high, without gonophores [N 1768]; Arembepe region, 22 m depth, 10.xi.1996, three colonies, 6-6.5 mm high, male and female gonophores [N 1775]; Guarajuba Beach, on shallow bank coral reefs, 18 m depth, 16.viii.1996, fourteen colonies, 6.5-6.8 mm high, male and female gonophores [N 1788]; Praia do Forte Beach, on shallow bank coral reefs, 12 m depth, 24.iv.1997, eight colonies, 6.0-6.4 mm high, without gonophores [N 1792]; 21.ix.1997, 14 m depth, four colonies, 6.5-6.8 mm high, gonophores not observed [N 1795].
Description: Soft brown or yellowish stolonal colony, up to 6.8 mm high, arising from a creeping hydrorhiza. Long pedicels, 3.4-4.6 µm in length, 50-68 µm in diameter; pedicel base with 2, 3 or 4 annulations; perisarc thick. Hydrothecae strongly campanulate, 640-975 µm long, 275-398 µm wide at margin, 69-76 µm wide at basal annular thickening; hydrothecal walls with thin perisarc, convex just above basal chamber, quite straight elsewhere. Hydrothecal margin with 9, 11 or occasionally 12 (never 10) cusps separated by U-shaped conspicuous embayments. Each marginal tooth with a distal concavity. Hydrothecal wall scalloped in cross section, with an U-shaped pleat extending outwards from hydrothecal cavity between adjacent cusp; basal chamber cup-shaped, 18-23 slightly filiform tentacles, 0.65-0.68 mm long. Gonothecae, yellowish, dimorphic; both types born on stolon; short and conspicuous pedicel; terminal aperture wide. Male gonothecae short, 1.42-1.85 mm long, 0.28-0.33. mm in diameter; sub-cylindrical; irregularly sinuous in a loose succession of incomplete rings. Female gonothecae, long, 1.88-2.23 mm in length, 0.30-0.35 mm in diameter; asymmetric, broadest near base, truncate below, tapering gradually above; incompletely ringed and/or irregularly folded. Nematocyst compliment represented by microbasic b-mastigophores of two different sizes: (i) A-type: 5.5-7.2 µm x 1.5-2.6 µm; (ii) B-type: 7.5-9.8 µm x 2.2-2.8 µm. The A-type is abundant throughout the entire colony, especially on tentacles, whilst the B-type is less abundant and never occurs on tenta-cles. Pseudo-microbasic b-mastigophores (Öst-man, 1988), 14.4 -19.5 µm x 2.1-3.6 µm, were not abundant.
Known range: Brazil: first record. Circumglobal distribution: western Atlantic (Fraser 1944); eastern Atlantic (Cornelius 1982, 1995); Indian Ocean (Millard 1975); western Pacific (Hirohito 1983); eastern Pacific (Fraser 1948), Bermuda (Calder 1991).
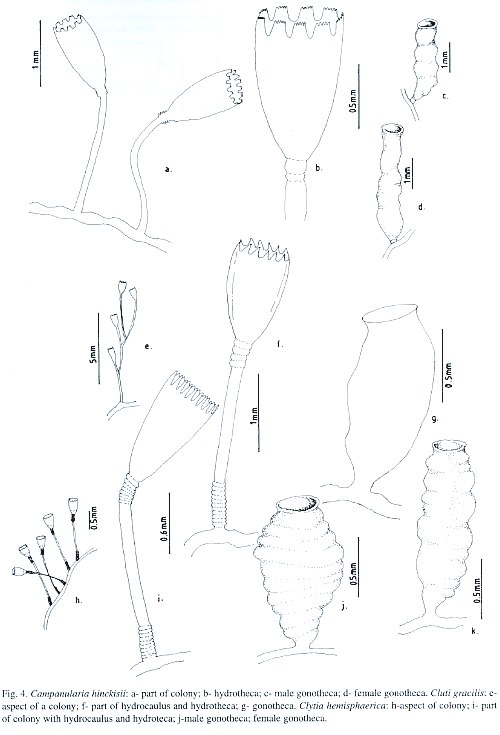
Laomedea gracilis Sars, 1850; Laomedea (Campanulariai) gracilis Sars, 1857; Clytia (Platypyxis) cylindrica L. Agassiz, 1862; Clytia cylindrica L. Agassiz, 1862; Platypyxis cylindrica L. Agassiz, 1862; Gonothyraea gracilis Allman, 1864; Campanularia gracilis van Beneden, 1867; Clytia laevis Weismann, 1883; Campanularia pelagica van Breemen, 1905; Campanularia (Clytia) pelagica van Breemen, 1905; Clytia coronata Fraser, 1912; Clytia elsae-oswaldae Stechow, 1914; Clytia pelagica Billard, 1917; Clytia gracilis Stechow, 1923c; Calder, 1991; Migotto, 1993; Cornelius, 1995; Laomedea (Phialidium) pelagica Hummelinck, 1930; Laomedea (Phialidium) gracilis Hummelinck, 1930; Laomedea pelagica Hummelinck, 1930; Laomedea (Clytia) gracilis Broch, 1933; Laomedea gracilis forma pelagica Leloup, 1933; Clytia (Campanularia) pelagica Künne, 1937; Laomedea cylindrica Leloup, 1937a; Clytia stechowi Hargitt, 1927; Clytia hemisphaerica Millard, 1966; Cornelius, 1982; Clytia elsaeoswaldae Vervoort, 1968; Campanularia (Clytia) cylindrica Vervoort, 1968; Clytia sarsi Cornelius, 1982.
Material studied: Todos os Santos Bay, Fontes Island, on the sponge Haliclona sp., 4.ix.1994, 1-2 m depth, six colonies, 4-8.5 mm high, gonophores observed [N 1753]; Itaparica Island, Mar Grande region, on coral reef building, 10.ix.1997, 5-6 m depth, nine colonies, 8 mm high, gonophores not seen [N 1898]; Salvador City, Ponta de Itapuã, on rock outcrop, 16.xii.1994, 18 m depth, nine colonies, 6-12 mm high, gonophores observed [N 1754]; Farol da Barra, on rock outcrop, 9.ii.1995, 6 m depth, eight colonies, 6-10.5 mm high, without gonophores [N 1761]; Ribeira Beach, on ascidians Styella plicata and Falusia nigra, 18.xi.1993, shallow subtidal, eleven colonies, 6.5-12 mm high, without gonophores [N 1762]; Northern Coast, Emissário beach, on internal beach rock bank, 15.xi.1995, shallow subtidal, twenty colonies, 4-8.5 mm high, without gonophores [N 1769]; Arembepe beach, on external beach rock bank, 15.xi.1995, 1-2 m depth, six colonies, 5-9.5 mm high, without gonophores [N 1771]; Arembepe beach, on embayment beach rock bank, 15.xi.1995, 2 m depth, thirteen colonies, 6-9 mm high, without gonophores [N 1772]; Arembepe region, on coral algal buildup, 20.xii.1992, 25 m depth, forty six colonies, 5-9 mm high, without gonophores [N 1774]; 17.iii.1993, 16 m depth, thirty four colonies, 6-10 mm high, gonophores observed [N 1782]; 24.ix.1996, 42 m depth, thirty one colonies, 4-6 mm high, gonophores not seen [N 1783]; Guarajuba beach, on coral reef wall, 4.xi.1995, 6-7 m depth, twenty six colonies, 8-12 mm high, gonophores observed [N 1784]; Itacimirim beach, on coral reef bank, 10.viii.1996, 11 m depth, ten colonies, 8-11 mm high, gonophores not observed [N 1785]; Praia do Forte beach, on shallow bank reef, 14.viii.1996, 18 m depth, thirty seven colonies, 6-10 mm high, gonophores not seen [N 1896].
Description: Colonies orange or dark golden, erect, fragile, sparingly and irregularly branched, up to 12 mm high, always arising from a creeping hydrorhiza. Hydrocaulus monosiphonic; each branch arising from a slightly curved aphophysis given off below hydrothecae of pedicel from which it arises. Branches resemble primary pedicels and directed abruptly upwards, 8-11 annulations proximally and 4 - 6 distally placed. Pedicels about 0.5-0.4 mm high, 101-142 µm in diameter, annulated basally and distally, with annuli or wrinkles elsewhere. Hydrothecae deeply cam-panulate, quite cone-shaped, about 727-935 µm long, 387-528 µm wide at margin, 159-241 µm wide at diaphragm, with thin perisarc. Walls convex above diaphragm, nearly straight elsewhere; margin with about 11 to 17 deeply cut, blunt, triangular cusps separated by U-shaped incisions. Diaphragm thin, straight; the basal chamber varied in depth but typically deep and cupshaped. Tentacles short, 18-21 in number, occasionally 24. Gonothecae, orange, urn-shaped, about 965-997 µm long, and 401-434 µm in diameter; usually symmetric; wide-mouthed, orifice diameter about 305-342 µm, constricted just below aperture to about 320 µm wide, arising from hydrorhiza on short inconspicuous pedicels; walls smooth, constricted below aperture. Nematocyst compliment comprising isorhizas about 5.9-6.5 x 1.5-1.9 µm and microbasic mastigophores of three distinct sizes; (i) small 6.3-8.0 x 1.9-2.5 µm; (ii) medium 8.5-9.3 x 2.8-3.2 µm; and (iii) large 13.6-16.8 x 3.3-4.1 µm.
Known range: Brazil: São Sebastião (São Paulo), Ponta do Jerobá, Parque de Cultivo de Mexilhões and Ponta do Baleeiro (Migotto, 1993). Circumglobal distribution: western Atlantic [Vervoort 1968, as Laomedea (Phialidium) pelagica]; eastern Atlantic (Vervoort 1946a, as Laomedea pelagica; Cornelius 1995); Indian Ocean (Mammen 1965); western Pacific (Yamada 1959); eastern Pacific (Fraser 1948, as Gonothyraea gracilis); Bermuda (Calder 1991).
Medusa hemisphaerica Linnaeus, 1767 [Medusa]; Sertularia uniflora Ellis, 1768; Sertularia volubilis Ellis and Solander, 1786; Oceania flavidula Péron and Lesueur, 1810; Oceania hemisphaerica Péron and Lesueur, 1810; Thaumantias hemisphaerica Eschscholtz, 1829; Campanularia johnstoni Alder, 1856; Campanularia gegenbaurii Sars, 1857; Campanularia volubilis Sars, 1857; Campanularia noliformis McCrady, 1859; Clytia bicophora L. Agassiz, 1862; Clytia (Trachopyxis) bicophoba L. Agassiz, 1862; Campanularia bicophora Allman, 1864; Clytia johnstoni A. Agassiz, 1865; Clytia (Campanularia) volubilis du Plessis, 1871; Campanularia (Clytia) johnstoni Hincks, 1872; Campanularia coronata Clarke, 1879; Epenthesis bicophora Haeckel, 1879; Clytia flavidula Metschnikoff, 1886; Campanularia edwardsi Nutting, 1901a; Clytia grayi Nutting, 1901a; Campanularia minuta Fraser, 1912b; Phialidium hemisphaericum Mayer, 1910; Campanularia (Clytia) johnstoni Broch, 1912; Clytia minuta Fraser, 1912b; Clytia uniflora Stechow, 1923a; Clytia similis Fraser, 1947; Phialidium noliformis Kramp, 1959; Phialidium noliforme Kramp, 1961; Campanularia (Clytia) coronata Vervoort, 1968; Clytia (Campanularia) hemisphaerica Gili, 1982; Clytia hemisphaerica Calder, 1991; Migotto, 1993; Cornelius, 1982, 1995.
Material studied: Todos os Santos Bay, Fontes Island, on Eudendrium carneum, 3.vi.1995, 1-2 m depth, nine colonies, up to 2 mm high, with male gonophores [N 1792]; Itaparica Island, Berlinque Beach, on shallow coral reef bank, 11.i.1993, 5 m depth, twenty one colonies, up to 8 mm high, with male and female gonophores [N 1790]; Salvador City, Boa Viagem beach, on rock outcrop, 23.v.1992, 8 m depth, five colonies, 6.5-9.4 mm high, without gonophores [N 1787]; Northern Coast, Emissário Beach, on internal beach rock bank, 15.xi.1995, intertidal zone, ten colonies, up to 4 mm high, with female gonophores [N 1793]; Arembepe Beach, on external beach rock bank, 15.xi.1995, 6 m depth, twelve colonies, up to 2.5 mm high, with female gonophores [N 1789]; Arembepe region, on coral algal buildups, 13.x.1996, 35 m depth, twenty three colonies, up to 3 mm high, with male and female gonophores [N 1786]; 10.ii.1997, 28 m depth, eighteen colonies, up to 3 mm high, gonophores not observed [N 1791].
Description: Colonies pale yellow to golden, particularly stolonal, arising from a creeping hydrorhiza. Tall hydrothecal pedicels, arising at close intervals. Hydrothecae of moderately thin perisarc, campanulated, urn-shaped, 587-976 µm long, 95-137 µm wide at diaphragm, 229-399 µm wide at margin; convex walls above diaphragm, nearly straight elsewhere. Pedicels usually straight, with 3-14 rings at top, and 2-11 at the base; specimens collected from rock out-crop with 8-9 central rings. Occasionally some pedicels with secondary pedicels arising from them. Hydrothecal margin with 13-17, but usually 14, rounded triangular cusps, separated by U-shaped incisions. Hydrothecal walls weakly scalloped in cross section at margin, with each cusp having a shallow, U-shaped pleat extending inwards towards hydrothecal cavity. Hydrothecal diaphragm quite thin and straight; basal chamber is rather deep and cup-shaped. Hydranth ovoid, with 20 to 32 thin tentacles, about 1.4 mm long. Gonothecae yellowish, dimorphic. Male gonotheca, 0.89-1.01 mm long, 0.45-0.61 mm wide, broad and asymmetric, with smooth walls and sub-terminal constriction; irregular annulations or folds at distal part; large terminal aperture, about 0.26 mm in diameter; pedicel short and usually inconspicuous. Female gonotheca, longer than male, 0.91-1.08 mm long, 0.42-0.46 mm wide, sub-cylindrical, distinct but irregular ribs; terminal aperture about 0.28 mm in diameter; short slender pedicel. Nematocyst compliment consists of microbasic b-mastigophores of two sizes: (i) A-type: 6.9-8.1 x 1.9-3.1 µm, throughout the colony; (ii) B-type: 15.3-17.5 x 3.5-4.2 µm, never occurs in tentacles.
Known range: Brazil: São Sebastião (São Paulo) (Migotto 1993). Circumglobal distribution: western Atlantic (Calder 1975); eastern Atlantic (Cornelius 1982, 1995); Indian Ocean (Mammen 1965); eastern Pacific (Fraser 1948, as C. johnstoni); Bermuda (Calder 1991).
Laomedea hummelincki Leloup, 1935; Campanularia hummelincki Fraser, 1944; Clytia hummelincki Millard, 1966; Calder, 1991; Migotto, 1993; Campanularia (Clytia) hummelincki Vervoort, 1968.
Material studied: Northern Coast, Emissário Beach, on internal beach rock bank, 15 th November 1995, 3 m depth, thirteen colonies, 3-4 mm high, with gonophores [N 1795]; Guarajuba Beach, on the sponge Dysidea variabilis from the shallow bank reef, 10 th April 1996, 19 m depth, three colonies, gonophores not seen [N 1796].
Description: Whitish or pale yellowish stolonal colonies, up to 4 mm high; creeping hydrorhiza. Pedicels very long, about 18-20 mm, thick perisarc, with 3-4 occasional groups of annuli irregularly placed along length; groups with 3-7 annuli; distal end of pedicel with slight swelling just beneath a sub-hydrothecal spherule. Hydrothecae short, 299-314 µm long, and wide, 310-323 µm at margin, and 160-169 µm at diaphragm; margin entire, usually parallel or slightly sinuous; diaphragm thin, delicate and quite oblique in lateral view; basal chamber short. Hydranth with subspherical hypostome and 18-20 soft tentacles, about 2.6 mm long. Gonothecae, whitish, sessile, truncate, pear-shaped and short, 0.76-0.88 mm long; about 0.23 mm in diameter at base and 0.39 at margin; usually symmetric; terminal aperture rounded and wide; two medusa buds insuccessive development. Nematocyst compliment represented by small microbasic b-mastigophores, 3.1-3.4 µm x 0.55-0.59 µm, throughout the colony.
Known range: Brazil: São Sebastião (São Paulo) (Migotto 1993). Circumglobal distribution: western Atlantic (Leloup 1935); eastern Atlantic (Millard 1975, Cornelius 1982); Bermuda (Calder 1991). Clytia linearis (Thornely, 1900) (Plate II c-f) Obelia linearis Thornely, 1900; Campanularia ravieri Billard, 1904; Campanularia linearis Borradaile, 1905; Obelia striata Clarke, 1907; Clytia fragilis Congdon, 1907; Clytia striata Vanhöffen, 1910; Clytia linearis Stechow, 1913; Laomedea striata Kramp, 1922; Clytia alternata Hargitt, 1924; Laomedea (Obelia) bistriata Leloup, 1931; Laomedea bistriata Leloup, 1932; Laomedea gravieri Billard, 1933; Laomedea tottoni Leloup, 1935; Laomedea fragilis Leloup, 1935; Clytia gravieri Billard, 1938; Clytia acutidentata Fraser, 1938; Clytia carinadentata Fraser, 1938; Gonothyraea serialis Fraser, 1938; Clytia obliqua Picard, 1950; Clytia serrata Millard, 1958; Campanularia (Clytia) gravieri Vervoort, 1967; Laomedea (Phialidium) tottoni Vervoort, 1968; Clytia linearis Calder, 1991; Migotto, 1993.
Material studied: Todos os Santos Bay, Pati Island, on the sponge Tedania ignis, intertidal zone, 2.vi.1995, two colonies, 5 mm high, gonophores observed [N 1794]; Mar Grande -Coroa Beach, on biogenic rock, 4m depth, 20.x.1994, 13 colonies, 10-12 mm high, gonophores observed [N 1805]; Salvador City, Farol da Barra, on rock outcrop, 17 m depth, 30.x.1993, five colonies, 10-14 mm high, with-out gonophores [N 1797]; Pituba Beach, on rock outcrop, 4-5 m depth, 15.viii.1996, seven colonies, 8 - 10 mm high, gonophores observed [N 1798]; Ribeira beach, on the bivalve Anomalocardia brasiliana, intertidal zone, 18.xi.1993, eight colonies, 9.5 - 13 mm high, gonophores observed [N 1799]; Northern Coast, Arembepe beach, on external beach rock bank, 8-9 m depth, 15.xi.1995, fourteen colonies, up to 6 mm high, gonophores observed [N 1781]; Arembepe region, on coral algal buildup, 38 m depth, 20.iv.1996, sixteen colonies, 5 - 6.5 mm high, gonophores not seen [N 1801]; 22 m depth, 14.vi.1996, twelve colonies, 6-6.5 mm high, gonophores not seen [N 1802]; Guarajuba Beach, on coral reef wall, 9 m depth, 12.iv.1996, eleven colonies, 8-10 mm high, gonophores not seen [N 1803]; Praia do Forte, on shallow bank reef, 16 m depth, 14.iv.1996, fifteen colonies, 8-10 mm high, gonophores not observed [N 1804].
Description: Stolonal or erect colonies, with brownish and thick perisarc. Stolonal forms, up to 6 mm high, arising from a creeping hydrorhiza; erect forms branching sympodially up to 13 mm high, but usually 10 mm. Hydrocaulus always unbranched, monosiphonic, divided by internodes at regular intervals. Internodes long, about 1.6 mm in length, slender, straight or sometimes slightly curved; a group of 6-9 annuli are basally or subbasally placed; distal end with a hydrothecal pedicel composed of 5-7 narrow annuli. All internodes with apophysis, except terminal ones. Apophysis short, curved upwards, alternating from side to side, each supporting an intern-ode. Hydrothecae strongly campanulated, long, 760-988 µm in length, 316-478 µm wide at margin and 145-199 µm wide at diaphragm, sometimes asymmetric. Hydrothecal margin with 9-16 long cusps, separated by U-shaped incisions; each cusp with internal stiffening strip reaching to tip and extending downwards sometimes to middle of hydrotheca; diaphragm thin and oblique, occasionally transverse; basal chamber cup-shaped, of variedsize. Hydranth more or less ellipsoid, with 10-16 long tentacles, about 465 mm long. Gonothecae cone-shaped. Young ones light brown, elongated, 650-712 µm long, pedicel of 3-4 rings, usually on stolon, but sometimes on erect shoot in axil; wide in centre, about 310 µm in diamenter, and sometimes at terminal end, tapering below, 230 µm wide; gonophores with 1 or 2 rows of developing medusae.
Mature gonotheca, dark orange, longer, 960-989 µm long, placed like the young ones; asymmetric; wide terminal orifice, about 330 µm in diameter, usually constricted just below margin; short pedicel, 4-6 annuli; 2-4 developing medusae. Nematocyst compliment (excluding medusae) represented by microbasic b-mastigophores of two different sizes: (i) A-type: 7.8-9.2 x 2.4-3.1 µm and, (ii) B-type: 12.2-13.5 x 3.5 - 4.4 µm.
Known range: Brazil: São Sebastião (São Paulo) (Migotto 1993). Circumglobal distribution: western Atlantic [Vervoort 1968, as Laomedea (Phialidium) tottoni]; eastern Atlantic (García, Aguirre and González 1978, as Clytia gravieri; Cornelius 1982); Indian Ocean (Millard 1975 as Clytia gravieri); western Pacific (Yamada 1959); eastern Pacific (Fraser 1938a, as C. acutidentata, C. carinadentata, and Gonothyraea serialis); Bermuda (Calder 1991).
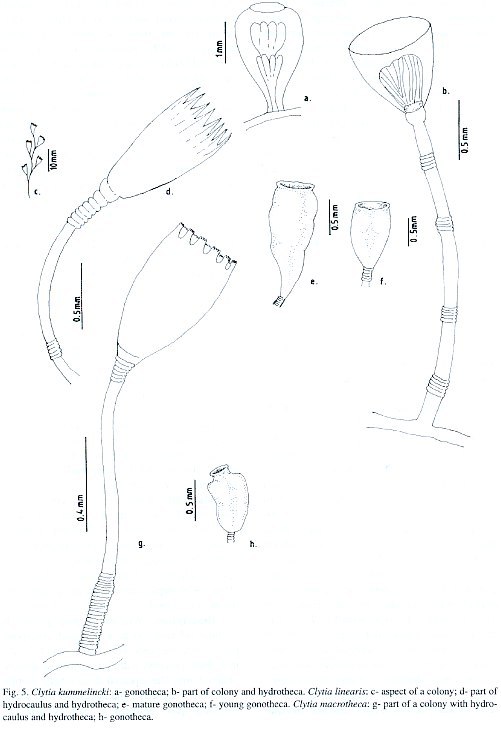
Campanularia macrotheca Perkins, 1908; Clytia macrotheca Stechow, 1923; Laomedea macrotheca Leloup, 1935; Campanularia (Clytia) macrotheca Vervoort, 1968; Clytia macrotheca Calder, 1991.
Material studied: Salvador City, Ribeira Beach, on the hydroid Thyroscyphus ramosus, 2 m depth, 18.xi.1993, eighteen colonies, 5mm high, gonophores observed [N 1806]; Northern Coast, Arembepe region, on embayment beach rock bank, 3-4 m depth, 15.xi.1995, three colonies, 3-3.5 mm high, gonophores not observed [N 1807]; on external beach rock bank, 6-8 m depth, 15.xi.1995, twenty four colonies, 3 mm high, gonophores observed [N 1808]; on coral algal buildup, 30 m depth, 4.iii.1994, eleven colonies, up to 2 mm high, gonophores not seen [N 1809]; 55 m depth, 16.v.1994, six colonies, up to 2.5 mm high, gonophores not seen [N 1810]; Guarajuba Beach, on shallow coral reef bank, 19 m depth, 30.iv.1996, seven colonies, 3-4 mm high, without gonophores [N 1811]; Papa Gente Beach, on coastal emergent reef wall, 5-6 m depth, 1.v.1997, twelve colonies, 3 mm high, without gonophores [N 1812].
Description: Whitish or sometimes yellowish stolonal colonies, up to 5 mm high, arising from a creeping hydrorhiza. Delicate pedicels, 0.8-5.0 mm high, 35-50 µm in diameter, with a group of 22-25 basally placed annuli, and 6-8 annuli at distal end. No subhydrothecal spherule. Perisarc relatively thick, usually yellowish. Hydrothecae champagne-glass-shaped, 440-581 µm long, cylindrical distally, slightly constricted basally, 160-199 µm at margin, and 48-83 µm at diaphragm; margin with 9-11 truncated cusps separated by U-shaped incisions. Hydrothecal diaphragm thin, usually horizontal, but sometimes oblique; basal chamber shallow and cupshaped. Hydranth ovoid, whitish, with 14 tentacles, each about 400 µm long. Gonothecae white, irregularly pear-shaped,and sometimes cone-shaped; always asymmetric; short, about 620 µm long, 200 µm in maximum diameter at distal end, tapering towards the base, and these about 138 mm; terminal aperture wide, about 190 µm in diameter, slightly constricted below margin; short pedicels with 3-5 annuli. Nematocyst compliment represented by microbasic b-mastigophores of two sizes: (i) A-type: 5.5-6.0 x 1.8-2.0 µm, very abundant throughout the colony, and (ii) B-type: 8.0-8.5 x 2.5-2.8 µm, less numerous than A-type.
Known range: Brazil: first record. Circumglobal distribution: western Atlantic (Fraser 1944); Bermuda (Calder 1991).
Clytia volubilis Marktanner-Turneretscher, 1890; Verluys, 1899; Hargitt, 1909; Clytia noliformis Nutting, 1901a; Wallace, 1909; Fraser, 1912a, 1918, 1921, 1943, 1944, 1946, 1947; Bennitt, 1922; Timmermann, 1932; Burkenroad, 1939; Picard, 1949; Behre, 1950; Deevey, 1950; Mammen, 1965; Rees and Thursfield, 1965; Rees and White, 1966; Gravier, 1970; Gosner, 1971; Morris and Mogelberg, 1973; Defenbaugh and Hopkins, 1973; Defenbaugh, 1974; Ryland, 1974; Boero, 1981a, 1981b, 1987a; Spracklin, 1982; Flórez González, 1983; Calder, 1986, 1991; Llobet, Gili and Barangé, 1986; Östman, Piraino and Roca, 1987. Clytia simplex Congdon, 1907; Campanularia (Clytia) noliformis Winge, 1923; Leloup, 1932; Germain, 1935; Vervoort, 1968; Campanularia noliformis McCrady, 1859; Clytia folleata Vannucci Mendes, 1946.
Material studied: Todos os Santos Bay, Fontes Island, on the octocoral Caryjoa riisei, 1-2 m depth, 20.viii.1994, nine colonies, 2 mm high, without gonophores [N 1813]; Pati Island, on the sponge Haliclona carbonaria, intertidal zone, 3.xii.1994, three colonies, 2 mm high, without gonophores [N 1814]; Berlinque Beach, on coral reef bank, 9 m depth, 22.xii.1993, twenty nine colonies, up to 3 mm high, female gonophores observed [N 1833]; Salvador City, Farol da Barra, on rock outcrop, 18 m depth, 1.iii.1993, four colonies, 2.5 mm high, without gonophores [N 1815]; Pituba Beach, on rock outcrop, 2-3 m depth, 10.v.1996, six colonies, 2 mm high, without gonophores [N 1816]; Ponta de Itapuã, on rock outcrop, intertidal zone, 30.i.1997, eight colonies, up to 3 mm high, male and female gonophores observed [N 1817]; Ribeira Beach, on ascidian Botrylloides niger, shallow subtidal zone, 14.ii.1995, eighteen colonies, up to 3 mm high, male and female gonophores observed [N 1818]; Porto da Barra, on ascidian Styella plicata, 6-8 m depth, 23.xi.1996, six colonies, up to 3 mm high, male gonophores observed [N 1819]; Northern Coast, Arembepe Beach, on external beach rock bank, 4-5 m depth, 15.xi.1995, one colony, 2 mm high, female gonophore observed [N 1820]; Arembepe region, on coral algal buildup, 25 m depth, 30.iii.1994, thirteen colonies, up to 2 mm high, without gonophores [N 1821]; 37 m depth, 18.vi.1992, twenty two colonies, up to 2 mm high, without gonophores [N 1822]; 50 m depth, 20.ix.1995, six colonies, up to 2 mm high, female gonophores observed [N 1823]; 42 m depth, 10.x.1996, seventeen colonies, up to 2 mm high, male and female gonophores observed [N 1824]; 17 m depth, 30.i.1997, nineteen colonies, up to 2 mm high, male and female gonophores observed [N 1825]; 22 m depth, 5.v.1997, thirty two colonies, up to 2 mm high, without gonophores [N 1826]; Guarajuba Beach, on shallow bank reef, 18 m depth, 18.iv.1996, twenty seven colonies, up to 3 mm high, without gonophores [N 1834]; Itacimirim beach, on coastal emergent reef wall, 4-5 m depth, 20.iv.1996, eleven colonies, 2- 2.5 mm high, without gonophores [N 1835].
Description: Yellowish stolonal colonies, up to 3 mm high, arising from a creeping hydrorhiza. Pedicels with thick perisarc, strong, straight or slightly sinuous, relatively short, 1.1-2.6 mm high, 65-88 µm in maximum diameter; group of 18-20 strong annuli placed basally, and of 20-22 distally; usually smooth in between, but occasionally slightly waved. Hydrothecae with thick perisarc, campanulate, moderately shallow, 400-520 µm deep, 350-465 µm wide at margin, 135-190 µm wide at diaphragm; margin with 14 or 15 blunt pyramidal cusps, separately by shallow U-shaped incisions. Hydrothecal diaphragm thick, pale or crystalline, usually straight; basal chamber sub-spherical and shallow. Hydranth yellow or pale, spherical, with 25-28 tentacles, about 810 µm long each. Gonothecae dimorphic, usually golden or brownish, both arising from hydrorhiza on short annulated pedicels. Male: sub-cylindrical, irregularly folded, and usually asymmetric; short, about 650 µm long, 250 µm in maximum diameter; terminal aperture about 180 µm wide, placed on a semi-tubular neck. Female, coneshaped, symmetric, longer, about 780 µm long, 410 µm in maximum diameter; terminal aperture about 220 µm wide, placed on a tubular neck. Nematocyst compliment is represented by microbasic b-mastigophores of two sizes: (i) A-type: 4.0-5.0 x 1.2-1.5 µm, very abundant throughout the colony, and (ii) B-type: 8.5-9.1 x 2.4-2.9 µm, less numerous than A-type.
nown range: Brazil: first record. Circumglobal distribution: western Atlantic (Fraser 1944); eastern Atlantic (Picard 1949); Indian Ocean (Mammen 1965); eastern Pacific (Fraser 1948); Bermuda (Congdon 1907, Bennitt 1922, Burkenroad 1939, Morris and Mogelberg 1973, Ryland 1974, Calder 1986a, 1991).
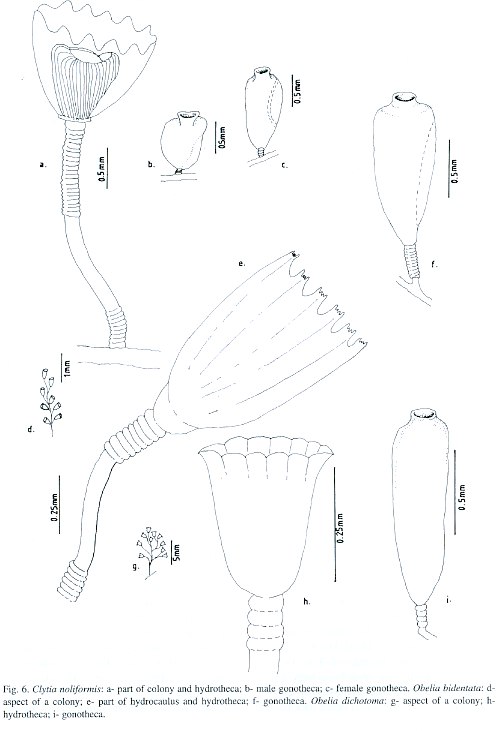
Obelia bidentata Clarke, 1875; Calder, 1991; Migotto, 1993; Cornelius, 1982, 1995; Obelia bicuspidata Clarke, 1875; Obelia longicyatha Allman, 1877; Obelia andersoni Hincks, 1887; Gonothyraea longicyatha Thornely, 1900; Clytia longicyatha Billard, 1906; Laomedea (Gonothyraea) bidentata Babic, 1913; Obelia spinulosa Annandale, 1915; Gonothyraea bicuspidata Stechow, 1919; Clytia longitheca Hargitt, 1924; Obelia longitheca Hargitt, 1924; Obelia atenuata Hargitt, 1924; Laomedea (Obelia) spinulosa var. minor Leloup, 1932; Laomedea spinulosa Leloup, 1933; Laomedea longicyatha Leloup, 1935; Laomedea (Obelia) biscuspidata Hummelincki, 1936; Laomedea bicuspidata Vervoort, 1946; Laomedea bicuspidata var. tenuis Vervoort, 1946; Laomedea (Obelia) longicyatha Vervoort, 1968.
Material studied: Salvador City, Farol da Barra, on rock outcrop, 18 m depth, 1.iii.1993, four colonies, 4 mm high, without gonophores [N 1836]; Northern Coast, Arembepe Beach, on external beach rock bank, 6-8 m depth, 15.xi.1995, eighteen colonies, up to 4 mm high, gonophores observed [N 1837]; Arembepe region, on coral-algal buildup, 37 m depth, 18.vi.1992, eleven colonies, 3-4 mm high, with-out gonophores [N 1838]; Guarajuba Beach, on coastal emergent reef wall, 6-8 m depth, 23.iv.1997, five colonies, 4 mm high, without gonophores [N 1839]; Praia do Forte Beach, on coastal emergent reef wall, 5-6 m depth, 25.iv.1997, twenty colonies, 3-4 mm high, with-out gonophores [N 1840].
Description: Brownish erect colonies, sympodial, up to 4 mm high, arising from a creeping hydrorhiza. Hydrocaulus unbranched or irregularly branched in older colonies, monosiphonic and straight, divided into internodes at more or less regular intervals. Perisarc thick. Internodes long, about 520 µm, with a group of 6 basally placed annuli; each internode supporting a hydrothecal pedicel from a distal apophysis; hydrothecal pedicel composed by 8-13 semi-spiral annulations, up to 200 µm in length; apophysis alternating from side to side. Hydrothecae deeply campanulated, semi-cylindric, 480-530 µm long, 167-210 µm wide at margin, and 60-83 µm wide at diaphragm; sometimes asymmetric; 6-8 conspicuous folds in hydrothecal walls runs down-wards from the margin; diaphragm always oblique; margin with 10-13 bimucronate cusps, never uniform, separated by U-shaped incisions. Hydrothecal diaphragm thin, delicate; basal chamber shallow, about 66-74 µm deep. Hydranth soft brown or pale, small, cylindrical, about 320 µm from diaphragm to tentacles. Tentacles short, 19-23 in number, about 265 µm long. Gonothecae coneshaped, pale to whitish, 760-830 µm long, 420-440 µm wide at distal end, tapering dowards the base, 55-70 µm wide, usually symmetrical. Terminal aperture, about 96 µm wide, placed on a collar neck. Base with a long pedicel, with 8-14 annulations. Nematocyst compliment represented by microbasic b-mastigophores A-type, 6.1-6.5 x 2.0-2.6 µm and by ishorhizas 5.1-6.5 x 1.1-2.0 µm.
Known range: Brazil: Baia de Guanabara (Vannucci 1949 as Gonothyraea bicuspidata); São Sebastião (São Paulo) (Migotto 1993). Circumglobal distribution: western Atlantic (Fraser 1944); eastern Atlantic (Cornelius 1975b, 1982, 1995); Indian Ocean (Mammen 1965); western Pacific (Hirohito 1983); eastern Pacific (Fraser 1937a); Bermuda (Calder 1991).
Sertularia dichotoma Linnaeus, 1758; Sertularia longissima Pallas, 1766; Laomedea (Sertularia) dichotoma Lamouroux, 1812; Sertularia geniculata Sprengel, 1813; Campanularia dichotoma Lamarck, 1816; Laomedea dichotoma Lamouroux, 1816; Campanularia maior Meyen, 1834; Campanularia brasiliensis Meyen, 1834; Campanularia cavolinii Deshayes and Milne-Edwards, 1836; Sertularia cavolini Kölliker, 1843; Laomedea dichotoma var. a Johnston, 1847; Campanularia (Laomedea) dichotoma Maitland, 1851; Obelia dichotoma Allman, 1864; Cornelius, 1982, 1995; Calder, 1991; Migotto, 1993; Eucope parasitica A. Agassiz, 1865; Eucope pyriformis A. Agassiz, 1865; Obelia hyalina Clarke, 1879; Obelaria dichotoma Haeckel, 1879; Obelaria (Campanularia) pyriformis Haeckel, 1879; Campanularia cheloniae Allman, 1888; Obelia angulosa Bale,1888; Laomedea (Obelia) dichotoma Levinsen, 1893; Obelia gracilis Calkins, 1899; Obelia surcularis Calkins, 1899; Obelia fragilis Calkins, 1899; Obelia griffini Calkins, 1899; Obelia rhunicola Billard, 1901; Obelia dubia Nutting, 1901; Campanularia obtusidens Jäderholm, 1904b; Obelia brasiliensis Hartlaub, 1905; Obelia congdoni Hargitt, 1909; Laomedea sargassi Broch, 1913; Obelia obtusidentata Bedot, 1925; Obelia everta Hargitt, 1927; Obelia alternata Fraser, 1938; Obelia equilateralis Fraser, 1938; Obelia microtheca Fraser, 1938; Obelia obtusidens Fraser, 1938; Obelia tenuis Fraser, 1938; Gonothyraea integra Fraser, 1940; Obelia biserialis Fraser, 1948; Laomedea (Obelia) congdoni Vervoort, 1968; Laomedea (Obelia) equilateralis Vervoort, 1968; Obelia (Laomede a) dichotoma Gili, 1982.
Material studied: Todos os Santos Bay, Fontes Island, on Eudendrium carneum, 1-2 m depth, 3.vi.1995, four colonies, 4-5 mm high, without gonophores [N 1827]; Northern Coast, Arembepe region, 50 m depth, 20.ix.1995, eight colonies, 6 mm high, gonophores observed [N 1828]; 25 m depth, 20.xii.1992, eighteen colonies, 5-6 mm high, gonophores observed [N 1829]; 42 m depth, 24.ix.1996, twenty colonies, 5-6 mm high, gonophores observed [N 1830]; Northern Coast, Itacimirim Beach, on shallow bank reef, 15 m depth, 20.iv.1996, fifteen colonies, 10-12 mm high, ithout gonophores [N 1831]; Praia do Forte Beach, on coastal emergent reef wall, 5-6 m depth, 25.iv.1997, twenty three colonies, up to 12 mm high, without gonophores [N 1832].
Description: Soft orange, brown, pale or slightly pinkish erect or bushy colonies, up to 12 mm high, sympodial, arising from a creeping hydrorhiza. Hydrocaulus usually branched, monosiphonic, divided into internodes at regular intervals. Internodes varied in length, slightly curved, slender, annulated basally, about 5-8 annuli, supporting a hydrothecal pedicel from distal apophysis. Hydrothecal pedicels with 4, 5 or 8 annulations. Hydrothecae, wide, bell-shaped, 250-280 µm long, 200-245 µm wide at margin, 95-110 µm wide at diaphragm; margin slightly crenulate, 14-16 soft cusps, and 13-15 fine longitudinal folds, or sometimes entire; diaphragm thin, transverse or slightly oblique; basal chamber usually small. Hydranth short, cylindrical, about 150 µm long, with 25-38 tentacles, each 220-240 µm long. Gonothecae, whitish or yellowish, conical, long, 820-912 µm long, 240-300 µm in maximum diameter at distal end, tapering basally, 112-154 µm wide. Terminal aperture on a tubular neck. Pedicels short, 30-42 µm long, sometimes with 4-5 annulations, and usually arising directly from hydrothecal pedicel. Nematocyst compliment represented by microbasic b-mastigophores of small size, 4.8-7.5 x 1.9-2.5 µm on tentacles and hypostome. Isorhizas of two different sizes: (i) 8.0-9.1 x 1.6-2.0 µm, not recorded from tentacles and, (ii) 5.1-8.0 x 1.0-1.6 µm less numerous throughout the colony.
Known range: Brazil: Rio de Janeiro (Stechow 1919 as O. angulosa); São Sebastião (São Paulo) (Migotto 1993). Circumglobal distribution: western Atlantic (Fraser 1944); eastern Atlantic (Cornelius 1975a, 1982, 1995); Indian Ocean (Millard 1975); western Pacific (Yamada 1959); eastern Pacific (Fraser 1937); Bermuda (Congdon 1907, Bennitt 1922, Calder 1986, 1991).
Discussion
Three distinct groups of hydroids were separated based upon species composition and geographical location along the coast of Bahia. The first group, represented by sandy shores, was characterised solely by three species of Clytia, of which two (C. macrotheca and C. noliformis) are recorded for the first time from Brazil. The second cluster comprised mangroves and rocky shores, and was characterised by five species, including C. gracilis and C. noliformis. The third group (beach rocks, coral-algal buildup and coral reefs) was located at the northern coast of Bahia, and had higher species numbers than the other two groups, including the presence of Campanularia hincksii (recorded for the first time in Brazil).
Phenotypically plastic species such as Clytia gracilis and C. noliformis colonised all six ecosystems assessed, including sandy shores where only three hydroid species were recorded. This wide distribution a wide tolerance to various levels of physical stress as long as there is a hard substratum for attachment. In contrast, C. hummelincki, a remarkably rare species (Cornelius 1982, Calder 1991, Migotto 1993), occurred in smaller numbers and was recorded only from two ecosystems, each with oligotrophic waters. This indicates that this species requires very specific environmental conditions and may be lost if the ecosystem becomes further degraded.
Based on these distribution, it appears that hydroid species may be suitable candidates for biomonitors of Brazilian waters.
The campanulariids recorded from northern Bahia, and those from the south coast of Brazil (Migotto 1993), are usually smaller than those reported from Europe (Cornelius 1982, 1995). Morphological variations (i.e. margin of the hydrotheca, number of annulations throughout the colony, and abundance of certain types of nematocyst) can be taxonomically frustrating and make identifications difficult. However, these variations appear to be phenotypic responses to environmental conditions recorded in different ecosystems (Gili and Hughes 1995). Biochemical systematical studies are helpful in defining species, but to address the reasons behind such phenotipic variation requires detailed and intensive studies of hydroid communities, including quanti-tative and behavioural ecology.
Acknowledgements
The authors wish to thank the Environmental Protection Corporation (CETREL S/A), Petroleum of Brazil (PETROBRÁS) and Centro de Apoio ao Desenvolvimento Científico e Tecnológico (CNPq-Brazil) for financial support. The constructive criticism of the manuscript by two external referees P. Cornelius (Natural History Museum, London) and W. Vervoort (Nationaal Natuurhistorisch Museum, Leiden) are gratefully acknowledged. Sincere thanks are due to Malcolm B. Jones (Department of Biological Sciences, University of Plymouth) who kindly served as manuscript editor. Gratitude is extended to José María Landín Domínguez -Laboratory of Coastal Studies, and to Marlene Campos Peso Aguiar - Laboratory of Malacology and Benthic Ecology, both from Universidade Federal da Bahia, Brazil, for the technical facilities provided during the period of this research. Gratitude is expanded to Arilma Farias de Souza, Carlos Neves, Dirlene Cairo Aguiar, Larissa de Siqueira, Rita de Cássia Farani Assis, Rilza da Costa Tourinho Gomes, Simone Souza de Moraes, Tania Kobler Brazil and Yonara Souza Braga for all encouragement and assistance. Thanks to Tendai Mutyasera for the illustrations.
Resumen
La familia Campanulariidae es relativamente bien conocida, sin embargo varios aspectos de su biología y ecología no han sido aclarados. Este estudio presenta el primer enfoque semicuantitativo sobre los hidroidos campanularidos del noreste del Brasil, distribuidos hasta los 60 metros de profundidad y se basa en colecciones obtenidas desde 1992. Las colonias fueron colectadas de seis hábitats a lo largo de la costa de Ciudad de Salvador, Bahía de Todos os Santos, Isla de Itaparica hasta la región norte de la costa del estado de Bahia. Se registraron nueve especies de las 982 colonias examinadas: Campanularia hincksii, Clytia gracilis, C.hemisphaerica, C. Hummelincki, C. Linearis, C. Macrotheca, C. Noliformis, Obelia bidentata y O. Dichotoma. En la escala de abundancia Clytia gracilis y C. Noliformis fueron las especies más abundantes, mientras que Campanularia hincksii y Clytia hummelincki fueron las especies raras. El análisis de clasificación con datos de abundancia relativa mostró que las costas arenosas poseen una comunidad de hidroidos muy diferente a la de los otros hábitats. Se proporciona una clave simplificada de identificación, redescripciones, ilustraciones y un estudio del cnidoma para cada especie. Campanularia hincksii, Clytia macrotheca y C. noliformis se informan por primera vez para Brasil.
References
Adams, J. 1960. Acontribution to the biology of postlarval development of the Sargassum fish, Histrio histrio (Linnaeus), with a discussion of the Sargassum complex. Bull. Mar. Sci. Gulf Caribb. 10: 55-82. [ Links ]
Agassiz, A. 1865. Illustrated catalogue of the Museum of Comparative Zoology, at Harvard College. No. II, North American Acalephae. Cambridge, 234 p. [ Links ]
Agassiz, L. 1862. Contributions to the natural history of the United States of America, 4. Little Brown, Boston, 380 p. [ Links ]
Alder, J. 1856. Anotice of some new genera and species of British hydroid zoophytes. Ann. Mag. Nat. Hist. (Series 2). 18: 353-362.
Allman, G.J. 1864. On the construction and limitation of genera among the Hydroida. Ann. Mag. Nat. Hist. (Series 3). 13: 345-380. [ Links ]
Allman, G.J. 1877. Report on the Hydroida collected during the exploration of the Gulf Stream by L.F.Portualès, assistant, United States Coast Survey. Mem. Mus. Comp. Zool. Harvard Coll. 5:1-66.
Allman, G.J. 1888. Report on the Hydroida dredged by H.M.S. Challenger during the years 1873-76. Part II: The Tubularinae, Corymorphinae, Campanularinae, Sertularinae, and Thalamophora. Report on Scientific Results of the Voyage H.M.S. Challenger during the years 1873-76. Zoology. 7: 1-90.
Annandale, N. 1915. Fauna of the Chilka Lake. The coelenterates of the lake, with an account of the Actiniaria of brackish water in the Gangetic delta. Mem. Indian Mus. 5: 65-114. [ Links ]
Babic, K. 1913. Bemerkungen zu den zwei in der Adria vorkommenden thacaphoren Hydroiden. Zool. Anz. 43: 284-288. [ Links ]
Bale, W.M. 1887. The genera of the Plumulariidae, with observations on various Australian hydroids. Trans. and Proc. Royal Soc. Victoria. 23: 73-110. [ Links ]
Bale, W.M. 1888. On some new and rare Hydroids in the Australian Museum Collection. Proc. Lin. Soc. New South Wales. 2(3): 745-799. [ Links ]
Bedot, M. 1925. Matériaux pour servir a lhistoire des hydroïdes. 7éme période (1901 à 1910). Rev. Suisse Zool. 32: 1-657. [ Links ]
Behre, E.H. 1950. Annotated list of the fauna of the Grand Isle region 1928-1946. Occasional Papers of the Marine Laboratory, Louisiana State Univ. 6: 1-66.
Bennitt, R. 1922. Additions to the hydroid fauna of the Bermuda. Proc. Amer. Acad. of Arts Sci. 57: 241-259.
Billard, A.1901. De la stolonisation chez les hydroïdes. Comptes rendus Hebdomadaires des Séances lAcad. Sci. 133: 521-524. [ Links ]
Billard, A. 1904. Contribution à létude des hydroïdes (multiplication, régénération, greffes, variations). Ann. Sci. Natur., Zool. Paléontol. (8) 20: 1-251. [ Links ]
Billard, A. 1906. Note sur les hydroïdes du Travailler et du Talisman. Bull. Mus. dHist. Natur. 12: 329-334.
Billard, A. 1917. Note sur quelques espèces dhydroïdes libres. Bull. Mus. Nat. dHist. Natur. 23: 539-546.
Billard, A. 1933. Les hydroïdes des Golfes de Suez et dAkaba. Mém. lInst. dÉgypt. 21: 1-30.
Billard, A. 1938. Note sur une espèce de campanularidés (Clytia gravieri, Billard). Bull. Mus. Nat. dHist. Natur. Series 2. 10: 429-432. [ Links ]
Boero, F. 1981a. Systematics and ecology of the hydroid population of two Posidonia oceanica meadows. Mar. Ecol. 2: 181-197.
Boero, F. 1981b. Osservazioni ecologiche sugli idroidi della fascia a mitili della Riviera Ligure di levante. Cahiers Biol. Mar. 22: 107-117.
Boero, F. 1987. Evolutionary implications of habitat selection in the hydroids of Posidonia oceanica meadows. In J. Bouillon, Boero, F., Cicogna, F. & P.F.S. Cornelius (eds.). Modern trends in the systematics, ecology, and evolution of hydroids and hydromedusae. Claredon, Oxford. pp. 251-256. [ Links ]
Borradaile, L.A. 1905. Hydroids. The Fauna and Geography of the Maldive and Laccadive Archipelagos. 2: 836-845. [ Links ]
Bouillon, J. 1985. Essai de classification des hydropolypes-hydroméduses (Hydrozoa-Cnidaria). Indo Malayan Zool. 1: 29-243. [ Links ]
Bray, J.R. & Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 27: 325-349. [ Links ]
Broch, H. 1912. Hydroiduntersuchungen III. Vergleichende Studien an Adriatischen Hydroiden. Det Kongelige Norske Videnskabers Selskabs Skrifter. 1: 1-65. [ Links ]
Broch, H. 1913. Hydroida from the "Michael Sars" North Atlantic Deep Sea Expedition 1910. Report on the Scientific Results of the "Michael Sars" North Atlantic Deep Sea Expedition 1910. Zoology 3: 1-18. [ Links ]
Broch, H. 1933. Zur Kenntnis des Adriatischen Hydroidenfauna von Split. Arten und Variationen. Skrifter Utgitt av det Norske Videnskaps-Akademi I Oslo I, Matematisk-Naturvidenskapelig Kl. 4: 1-115.
Burkenroad, M.D. 1939. In Parr, A.E. Quantitative observations on the pelagic Sargassum vegetation of the western north Atlantic. Bull. Bingham Oceanogr. Collec. 6: 1-94.
Calder, D.R. 1975. Biotic census of Cape Cod Bay: Hydroids. Biol. Bull. Mar. Biol. Lab. Woods Hole. 149: 287-315. [ Links ]
Calder, D.R. 1986. Class Hydrozoa. pp. 127-155. In W. Sterrer (ed.). Marine fauna and flora of Bermuda: a systematic guide to the identification of marine organisms. Wiley-Interscience, New York. [ Links ]
Calder, D.R. 1988. Shallow water hydroids of Bermuda: the Athecatae. Roy. Ontario Mus., Life Sci. Contrib. 148. 107 p. [ Links ]
Calder, D.R. 1991. Shallow water hydroids from Bermuda: the Thecatae, exclusive of Plumularioidea. Roy. Ontario Mus. Life Sci. Contrib. 154. 140 p. [ Links ]
Calder, D.R. 1997. Synopsis of hydroids from 1000 m and deeper in the western North Atlantic. In J.C. den Hartog et al. (eds.). Proceedings of the 6 th International Conference on Coelenterate Biology: 85-90. Nationaal Natuurhistorisch Museum, Leiden, The Netherlands: i-xviii, 1-542.
Calkins, G.N. 1899. Some hydroids from Puget Sound. Proc. Boston Soc. Nat. Hist. 28: 333-368.
Carr, M.R. 1996. Plymouth Routines in Multivariate Analysis. Primer user manual. 45 p.
Clarke, S.F. 1875. Descriptions of the new and rare species of hydroids from the new England coast. Trans. Connecticut Acad. Arts Sci. 3: 58-66. [ Links ]
Clarke, S.F. 1879. Report on the Hydroida collected during the exploration of the Gulf Stream and Gulf of Mexico by Alexander Agassiz, 1877-88. Bull. Mus. Comp. Zoöl. Harvard Coll. 5: 239-252. [ Links ]
Clarke, S.F. 1907. Reports on the scientific results of the expedition to eastern tropical Pacific, in charge of Alexander Agassiz, by the U.S. Fish Commission steamer "Albatross", from October, 1904, to March, 1905, Lieut. – Commander L.M. Garret, U.S.N., commanding. VIII. The hydroids. Mem. Mus. Comp. Zool. Harvard Coll. 35(1): 1-18.
Congdon, E.D. 1907. The hydroids of Bermuda. Proc. Of the American Acad. of Arts and Sci. 42: 463-485.
Cornelius, P.F.S. 1975a. A revision of the species of Lafoeidae and Haleciidae (Coelenterata:Hydroida) recorded from Britain and nearby seas. Bull. of the Brit. Mus. (Natur. Hist.). Zool. 28: 375-426. [ Links ]
Cornelius, P.F.S. 1975b. The hydroid species of Obelia (Coelenterata, Hydrozoa, Campanulariidae), with notes on the medusa stage. Bull. Brit. Mus. (Natur. Hist.). Zool. 28: 249-293. [ Links ]
Cornelius, P.F.S. 1982. Hydroids and medusae of the family Campanularidae recorded from the eastern North Atlantic, with a world synopsis of genera. Bull. Brit. Mus. (Natur. Hist.). Zool. 42: 37-148. [ Links ]
Cornelius, P.F.S 1995. North-west European thecate hydroids and their medusae (Cnidaria, Leptolida, Leptothecatae). Part 2. Synopses of the British Fauna, 50. 396 p.
Deevey, E.S. 1950. Hydroids from Louisiana and Texas, with remarks on the Pleistocene biogeography of the western Gulf of Mexico. Ecology 31: 334-367.
Defenbaugh, R. 1974. Hydroids. pp. 94-112. In T.J. Bright & L.H. Pequegnat (eds.). Biota of the West Flower Garden Bank. Gulf Publishing, Houston. [ Links ]
Defenbaugh, R. & S.H. Hopkins. 1973. The occurrence and distribution of the hydroids of the Galveston Bay, Texas area. Texas A & M University, TAMU-SG-73-210. 202 p. [ Links ]
Deshayes, G.P. & H. Milne-Edwards. 1836. Histoire naturelle des animaux sans vertèbres par J.B.P.A. de Lamarck, 2. Baillière, Paris, 683 pp. [ Links ]
Ellis, J. 1768. An account of the Actinia sociata, or clustered animal-flower, lately found on the sea-coasts of new ceded islands. Phil. Trans. Roy. Soc. London. 57: 428-437. [ Links ]
Ellis, J. & Solander, D. 1786. The natural history of many curious and uncommon zoophytes, collected from various parts of the globe by the late J. Ellis Systematically arranged and described by the late Daniel Solander. London. 206 p.
Eschscholtz, F. 1829. System der Acalephen. Eine aus-führliche Beschreibung aller medusenartigen Strahltie. Ferdinand Dümmler, Berlin, 190 p.
Flórez González, L. 1983. Inventário preliminar de la fauna hydroide de la Bahía de Cartagena y áreas adyacentes. Bol. Mus. Mar. 11: 385-391.
Fraser, C.M. 1912. Some hydroids of Beaufort, North Carolina. Bull. U. S. Bur. Fisheries. 30: 339-387.
Fraser, C.M. 1918. Hydroids of eastern Canada. Contrib. Canadian Biol. 1917-1918, pp. 329-367.
Fraser, C.M. 1921. Key to the hydroids of eastern Canada. Contrib. Canadian Biol. 1918-1920. 137-180. [ Links ]
Fraser, C.M. 1937. Hydroids of the Pacific coast of Canada and the United States. University of Toronto, Toronto, 207 p. [ Links ]
Fraser, C.M. 1938. Hydroids of the 1934 Allan Hancock Pacific Expedition. Allan Hancock Expeditions. 4: 1-105.
Fraser, C.M. 1940. Seven new species and one new genus of hydroids, mostly from the Atlantic Ocean. Proc. U. S. Nat. Mus. 88: 575-580.
Fraser, C.M. 1943. Distribution records of some hydroids in the collection of the Museum of Comparative Zoölogy at Harvard College, with description of new genera and new species. Proc. New England Zool. Club. 22: 75-98. [ Links ]
Fraser, C.M. 1944. Hydroids of the Atlantic coast of North America. University of Toronto. Toronto. 451 p. [ Links ]
Fraser, C.M. 1946. Distribution and relationship in American hydroids. University of Toronto, Toronto. 464 p.
Fraser, C.M. 1947. Hydroids of the 1939 Allan Hancock Caribbean Sea Expedition. Allan Hancock Atlantic Expedition. 4: 1-24.
Fraser, C.M. 1948. Hydroids of the Allan Hancock Pacific Expeditions since March, 1938. Allan Hancock Pacific Expeditions. 4: 179-335.
García-Corralez, P., A. Aguirre-Inchaurbe & D. González Mora. 1978. Contribuición al conocimiento de los hidrozoos de las costas Españolas. Parte I: halécidos, campanuláridos y plumuláridos. Bol. Inst. Español Oceanogr. 4: 3-73. [ Links ]
Germain, L. 1935. La mer de sargasses. Bull. lInst. Océanogr. 671: 1-23. [ Links ]
Gili, J.M. 1982. Fauna de cnidaris de les illes Medes. Treballs Inst. Catalana dHist. Natur. 10, 175 p.
Gili, J.M. & R.G. Hughes. 1995. The ecology of marine benthic hydroids. A.D. Ansell, R.N. Gibson & M. Barnes (eds.). Oceanog. Mar. Biol.: Annu. Rev. 33:351-426.
Gosner, K.L. 1971. Guide to identification of marine and estuarine invertebrates. Wiley-Interscience, New York. 693 p. [ Links ]
Gravier, N. 1970. Étude des hydraires epiphytes des phanérogames marines de la région de Tulear (sud-ouest de Madagascar). Recueil des Travaux de la Station Marine dEndoume, Fascicule Hors Série Supplément. 10: 111-161.
Haeckel, E. 1879. Das System der Medusen. Erster Theil einer Monographie der Medusen. Denksch. Med.-Naturwiss. Ges. Jena 1. 360 p. [ Links ]
Hargitt, C.W. 1909. New and little known hydroids of Woods Holl. Biol. Bull. 17: 369-385. [ Links ]
Hargitt, C.W. 1924. Hydroids of the Philippine Islands. Philippine J. Sci. 24: 467-507. [ Links ]
Hargitt, C.W. 1927. Some hydroids of South China. Bull. of the Mus. Comp. Zoöl. Harvard Coll. 67: 491-520. [ Links ]
Hartlaub, C. 1905. Die Hydroiden der magalhaensischen Region und chilenischen Küste. Zool. Jahrbücher. Sappl. 6:497-714. [ Links ]
Hincks, T. 1853. Further notes on British zoophytes, with descriptions of new species. Ann. Mag. Natur. Hist. (Series 2). 11: 178-185. [ Links ]
Hincks, T. 1872. Note on Prof. Hellers Catalogue of the Hydroida of the Adriatic. Ann. Mag. Natur. Hist. (Series 4). 9: 116-121. [ Links ]
Hincks, T. 1877. Contributions to the history of the Hydroida. Ann. Mag. Natur. Hist. (Series 4). 19: 148-152.
Hirohito, his majesty the showa emperor. 1983. Hydroids from Izu Ôshima and Niijima. Tokyo, Biological Laboratory, Imperial Household, (6): 83 p.
Hummelinck, P.W. 1930. Beiträge zur Kenntnis Holländischer Hydroiden I. Bemerkungen über einige Campanuliden und Campanulariden vom Vagdam und Nieuwediep. Tijdschr. Nederland. Dierkundige Vereeniging. (Series 3). 2: 28-42.
Hummelinck, P.W. 1936. Hydropoliepen. Flora en Fauna der Zuiderzee. Suppl. Zuiderzee-Comm. Nederland. Dierkundige Vereeniging. pp. 41-64. [ Links ]
Jäderholm. E. 1904. Hydroiden aus den Küsten von Chile. Ark. Zool. 2: 1-7. [ Links ]
Johnston, G. 1847. A history of the British zoophytes, 2 vols. John van Voorst, London. 488 p. [ Links ]
Kelmo, F. & S. Peixinho. 1996. Hydroids as indicators of coral reef health. Abstr. 8 th Int. Coral Reef Symp. Panama City, Panama. 96 p.
Kelmo, F. & L.M. Santa-Isabel. 1998. Athecatae hydroids (Cnidaria-Hydrozoa) from Northern Bahia, Brazil. Rev. Biol. Trop. 46(5): 61-72.
Kölliker, A. 1843. Ueber die Randkörper der Quallen, Polypen und Strahlthiere. Nueu Notizen aus dem Gebiete der Natur- und Heilkund. 25: 81-84.
Kramp, P.L. 1922. Kinetocodium danae n.g., n.sp., a new gymnoblastic hydroid. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening i Kjøbenhavn. 74:1-21.
Kramp, P.L. 1959. The hydromedusae of the Atlantic Ocean and adjacent waters. Dana Rep. 46: 1-283. [ Links ]
Kramp, P.L. 1961. Synopsis of the medusae of the world. J. Mar. Biol. Ass. UK. 40: 1-469. [ Links ]
Künne, C. 1937. Über die Verbreitung der Leitformen des Grossplanktons in der südlichen Nordsee im Winter. Ber. Deutschen Wiss. Komm. Meeresforsc. 8:131-164.
Lamarck, J.B. P.A. 1816. Histoire naturelle des animaux sans vertèbres. Tome 2. Paris, Verdière. 568 p. [ Links ]
Lamouroux, J.V.F. 1812. Extrait dun memoire sur la classification des polypieres coralligènes non entièrement pierreux. Nouv. Bull. Sci. Soc. Philom. Paris. 3:181-188. [ Links ]
Lamouroux, J.V.F. 1816. Histoire des polypieres coraligènes flexibiles, vulgairement nommés zoophytes. Caen, F. Poisson. 559 p.
Leloup, E. 1931. Trois nouvelles espèces dhydropolypes. Bull. Mus. Roy. Hist. Natur. Belg. 7(25): 1-6. [ Links ]
Leloup, E. 1932. Une collection dhydropolypes appartenant lIndian Museum de Calcutta. Rec. Indian Mus. 34: 131-170.
Leloup, E. 1933. Contribution à la connaissance des hydropolypes de la côte des Pays-Bas. Bulletin du Mus. Roy Hist. Natur. Belg. 9(45): 1-30.
Leloup, E. 1935. Hydraires calyptoblastiques des Indes Ocidentales. Mém. Mus. Roy. Hist. Natur. Belg. (2)2: 1-73. [ Links ]
Leloup, E. 1937. Résultats scientifiques des croisières du navire-école belge "Mercator". VI. Hydroidea, Siphonophora, Ceriantharia. Mém. Mus. Roy. Hist. Natur. Belg. (2)9: 91-121.
Levinsen, G.M.R. 1893a. Meduser, Ctenophorer og Hydroider fra Grønlands Vestkyst, tilligemed Bemaerkninger om Hydroidernes Systematik. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening i Kjøbenhavn. 5: 143-220.
Linnaeus, C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species cum characteribus, differentis, synonymis, locis. Holmiae, Laurentii Salvi. 823 p.
Linnaeus, C. 1767. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differenttis, synonymis, locis. Tomus I. Pars II. Holmiae, Laurentii Salvii, pp. 533-1317.
Llobet, I.; J.M.Gili & M. Barangé. 1986. Estudio de una poblacíon de hidropólipos epibiontes de Halimeda tuna. Misc. Zool. 10: 33-43.
Maitland, R.T. 1851. Descriptio systematica animalium, Belgii septentrionalis adjectis synonimis nec non locis in quibus reperiuntur, secundum classificationem Professoris J. van der Hoeven disposita. Leiden, C.C. van der Hoek. 234 p.
Mammen, T.A. 1965. On a collection of hydroids from south India. III. Family Plumulariidae. J. Mar. Biol. Assoc. India. 7: 291-324.
Marktanner-Turneretscher, G. 1890. Die Hydroiden des K.K. Naturhistorischen Hofmuseums. Ann. K.K. Naturhist. Hofmus. 5: 195-286. [ Links ]
Mayal, E.M. 1973. Hidróides (Hydrozoa, Hydroida) de Pernambuco. São Paulo. Instituto de Biociências, Universidade de São Paulo. 75 p. [ Links ]
Mayal , E.M. 1983. Distribuição dos hidróides (Hydrozoa, Thecata) na Costa do estado de Pernambuco, Brasil. Bol. Zool. Univ. São Paulo. 6: 1-13. [ Links ]
Mayer, A.G. 1910. Medusae of the world. The Hydromedusae. Carnegie Inst. Washington Pub. 109(2): 231-498. [ Links ]
McCrady, J. 1859. Gymnopthalmata of Charleston Harbor. Proc. Elliott Soc. Nat. Hist. 1: 103-221. [ Links ]
Metschnikoff, E. 1886. Medusologischen Mittheilung. Arb. Zool. Inst. Univ. Wien Zool. Sta. Triest. 6: 237-266. [ Links ]
Meyen, F.J.F. 1834. Beiträge zur Zoologie, gesammelt auf einer Reise um die Erde. Fünfte Abhandlung. Über das Leuchten des Meeres und Beschreibung einiger Polypen und anderer niederer Tiere. Novorum Actorum Naturae Curiosorum. 16: 125-216.
Migotto, 1993. Hidróides (Hydrozoa: Cnidaria) marinhos bentônicos da região costeira do canal de São Sebastião, SP. Universidade de São Paulo, São Paulo, Brazil. 258 p.
Migotto, A.E. & F.L. da Silveira. 1987. Hidróides (Cnidaria, Hydrozoa) do litoral sudeste e sul do Brasil: Halocordylidae, Tubulariidae e Corymorphidae. Iheringia Ser. Zool. 66: 95-115.
Millard, N.H.A. 1958. Hydrozoan from the coasts of Natal and Portuguese East Africa. Part I. Calyptoblastea. Ann. South African Mus. 44: 165-226. [ Links ]
Millard, N.H.A. 1966a. The Hydrozoa of south and west coasts of South Africa. Part III. The Gymnoblastea and small families of Calyptoblastea. Ann. South African Mus. 48: 427-487. [ Links ]
Millard, N.H.A. 1966b. Hydroids of the Vema Seamount. Ann. South African Mus. 50: 169-194. [ Links ]
Millard, N.H.A. 1975. Monograph on the Hydroida of southern Africa. Ann. South African Mus. 68: 1-513. [ Links ]
Morris, B.F. & D.D. Mogelberg. 1973. Identification manual to the pelagic Sargassum fauna. Bermuda Biological Sta. Res. Spec. Pub. 11: 1-63. [ Links ]
Narchi, W. & N.J. Hebling. 1975. The life cycle of the commensal hydromedusa Eutima sapinhoa n.sp. Mar. Biol. 30(1): 73-78. [ Links ]
Nutting, C.C. 1901a. The hydroids of Woods Hole region. Bull. U. S. Fish Comm. 19: 325-386.
Nutting, C.C. 1901b. Papers from Harriman Alaska Expedition. XXI. The hydroids. Proc. Washington Acad. Sci. 3: 157-216.
Östman, C. 1979a. Two species of nematocysts in Campanulariidae (Cnidaria, Hydrozoa) studied by light and scanning electron microscopy. Zool. Scripta. 8: 5-12. [ Links ]
Östman, C. 1979b. Nematocysts in the Phialidium medusae of Clytia hemisphaerica (Hydrozoa, Campanulariidae) studied by light and scanning electron microscopy. Zoon. 7: 125-142.
Östman, C. 1982a. Nematocysts and taxonomy in Laomedea, Gonothyraea and Obelia (Hydrozoa, Campanulariidae). Zool. Scripta. 11: 227-241. [ Links ]
Östman, C. 1982b. Isoenzymes and taxonomy in Scandinavian hydroids (Cnidaria, Campanulariidae). Zool. Scripta. 11: 155-163. [ Links ]
Östman, C. 1987. New techniques and old problems in hydrozoan systematics. In J. Bouillon, F. Boero, F. Cigona & P.F.S. Cornelius. (eds.). Modern Trends in the systematics, ecology, and evolution of hydroids and hydromedusae. Claredon, Oxford: pp. 67-82.
Östman, C. 1988. Nematocysts as taxonomic criteria with-in the family Campanulariidae, Hydrozoa. In Biology of nematocysts. Academic. pp. 501-517.
Östman, C., S. Piraino & I. Roca. 1987. Nematocyst comparisons between some Mediterranean and Scandinavian campanulariids (Cnidaria, Hydrozoa). In J. Bouillon, F. Boero, F. Cigona & P.F.S. Cornelius (eds.). Modern Trends in the systematics, ecology, and evolution of hydroids and hydromedusae. Claredon, Oxford: pp. 299-310.
Pallas, P.A. 1766. Elenchus zoophytorum sistens generum adumbrationes generaliores et specierum cognitarum succinctas descriptiones cum selectis auctorum synonymis. Hagae, Franciscum Varrentrapp. 451 p. [ Links ]
Peixinho, S. & M.C. Peso-Aguiar. 1989. Marine sponges as biomonitors: an approach at Todos os Santos Bay. Bahia, Brazil. Proc. Int. Workshop on Biomonitors. MARC UFBA, London.
Perkins, H.F. 1908. Notes on medusae of the western Atlantic. Papers from the Tortugas laboratory of the Carnegie Inst. Washington. 1: 135-149. [ Links ]
Péron, F. & C.A. Lesueur. 1810. Tableau des caractères génériques et spécifiques de toutes les espèces de méduses connues jusquà ce jour. Ann. Mus. Hist. Natur. 14: 325-366.
Petersen, K.W. 1979. Development of coloniality in Hydrozoa. In G. Lariwood & B.R. Rosen (eds.). Biology and Systematics of colonial organisms. Syst. Ass. Spec.Vol. 11: 105-139. [ Links ]
Petersen, K.W. 1990. Evolution and taxonomy in capitate hydroids and medusa (Cnidaria: Hydrozoa). J. Linn. Soc. London, Zool. 100: 101-31. [ Links ]
Picard, J. 1949. Sur la présence en Méditerrannée de Clytia noliformis (McCrady). Bull. Mus. Hist. Nat. Marseilles. 9: 184-190. [ Links ]
Picard, J. 1950. Observations sur les hydraires récoltés aux martigues dans le canal de Caronte. Vie et Milieu. 1:51-52. [ Links ]
Plessis, G. 1871. Évolution médusipare de Clytia (Campanularia) volubilis. Bull. Société vaudoise Sci. Nat. 11: 167-170.
Rees, W.J. & S. Thursfield. 1965. The hydroid collections of James Ritchie. Proc. Roy. Soc. Edinburg. 69: 34-220.
Rees, W.J. & E. White. 1966. New records and fauna list of hydroids from the Azores. Ann. Mag. Natur. Hist. 9: 271-284. [ Links ]
Ryland, J.S. 1974. Observations on some epibionts of gulf-weed, Sargassum natans (L.). Meyen. J. Exp. Mar. Biol. Ecol. 14: 17-25. [ Links ]
Sars, M. 1850. Beretning om en I Sommeren idhg foretagen zoologisk Reise i Lofoten og Finmarken. Nyt Mag. Naturvidensk. 6: 121-211. [ Links ]
Sars, M. 1857. Bidrag til kundskaben om Middelhavets Littoral-Fauna, Reisebemaerkninger fra Italien. Nyt Mag. Naturvidensk. 9: 110-164.
Silveira, F.L da. & A.E. Migotto. 1984. Serehyba sanctise-bastiani n.gen., n.sp. (Hydrozoa,Tubulariidae) simbiont of a gorgonian octocoral from the southeast coast of Brazil. Bijdr. Dierkd. 54(2): 231-242.
Silveira, F.L da. & A.E. Migotto. 1991. The variation of Halocordyle disticha (Cnidaria, Athecata) from the Brazilian coast: an environmental indicator species? Hydrobiologia 216/217: 437-442.
Silveira, F.L da. & A.E. Migotto. 1992. Rediscovery of Corymorpharia januarii Steenstrup, 1854 (Hydrozoa, Corymorphidae) on the southeastern and southern coasts of Brazil. Steenstrupia 18(4): 81-89.
Souza, A.F. 1997. Sertulariídeos (Hydroida-Thecatae) das construções recifais do litoral norte do estado da Bahia. Monografia de bacharelado. Instituto de Biologia, Universidade Federal da Bahia. 84 p.
Spracklin, B.W. 1982. Hydroidea (Cnidaria: Hydrozoa) from the Carrie Bow Cay, Belize. In K. Rützler & I.G. Macyntyre (eds.). The Atlantic barrier reef ecosystem at Carrie Bow Cay, Belize, I. Structure and communities. Smithsonian Contrib. Mar. Sci. 12: 239-251.
Sprengel, W. 1813. Philipp Cavolinis Abhandlungen über Pflanzen-Thiere des Mittelmeers. Nürnburg, J. Leonhard. 131 p. [ Links ]
Stechow, E. 1913. Hydropolypen der japanischen Ostküste II. Teil: Campanulariidae, Halecidae, Lafoeidae, Campanulidae und Sertularidae, nebst Ergänzungen zu den Athecata und Plumularidae. Abhandl. der Math.-Phys. Kla. Königlichen Bayerischen Akad. Wissenschften. Sappl. 3: 1-162.
Stechow, E. 1914. Zur Kenntnis neuer oder seltener Hydroidpolypen, meist Campanulariden, aus Amerika und Norwegen. Zool. Anzeiger. 45: 120-136.
Stechow, E. 1919a. Zur Kenntnis der Hydroidenfauna des Mittelmeeres, Amerikas und anderer Gebiete, nebst Angaben über einige Kirchenpauersche Typen von Plumulariidae. Zoologische Jahrbücher, Abt. Syst. Geog. Biol. Thiere. 42: 1-172.
Stechow, E. 1919b. Neue Ergebnisse auf dem Gebiete der Hydroidenenforschung. Münchener Med. Wochensch. 66: 852-853. [ Links ]
Thornely, L.R. 1900. The hydroid zoophytes collected by Dr. Willey in the southern seas. pp. 451-457. In A. Willey. Zoological results based on material from New Britain, New Guinea, Loyalty Islands and else-where, collected during the years 1895, 1896 and 1897. Part IV. Cambridge University, Cambridge.
Timmermann,G.1932. Biogeographische Untersuchungen über die Lebensgemeinschaft des treibenden Golfkrautes. Z. Morphol. Ökol. Tiere. 25: 288-335. [ Links ]
Van Beneden, P.J. 1867. Recherches sur la faune littorale de Belgique (polypes). Mém. Acad. Roy. Sci. Lettres Beaux-Arts de Belg. 36: 1-207. [ Links ]
Van-Breemen, P.J. 1905. Plankton van Noord- en Zuiderzee. Tjidschr. Nederland. Dierkunde Vereeniging (2). 9: 145-324. [ Links ]
Vanhöffen, E. 1910. Die Hydroiden der deutschen Südpolar-Expedition 1901-1903. Deut. Südpolar-Expedition 1901-1913, 11, Zool. 3: 269-340.
Vannucci, M. 1949. Hydrozoa do Brasil. Bol. Fac. Filosofia, Ciên. Letras, Univ. São Paulo, Zool. 14: 219-265.
Vannucci, M. 1950. Resultados Científicos do Cruzeiro do "Baependi" e do "Vega" a Ilha da Trindade. Hydrozoa. Bol. Inst. Ocean. S. Paulo. 1(1): 81-96.
Vannucci-Mendes, M. 1946. Hydroida Thecaphora do Brasil. Arq. Zool. Estado de São Paulo. 4: 535-538. [ Links ]
Verrill, A.E. 1873. Brief contributions to zoölogy, from the Museum of Yale College. Nº XXIV. Results of recent dredging expeditions on the coast of New England. Amer. J. Sci. Arts. 5: 98-106.
Versluys, J. 1899. Hydraires calyptoblastiques recueillis dans la mer des Antilles pendant lune des croisières accomplies par le comte R. de Dalmas sur son yacht CHAZALIE. Mém. Soc. Zool. France. 12: 29-58. [ Links ]
Vervoort, W. 1946. Hydrozoa (C 1). A. Hydropolypen. Fauna van Nederland, Aflevering. 14: 336 p. [ Links ]
Vervoort, W. 1967. The Hydroida and Chondrophora of the Israel South Red Sea expedition, 1962. Bull. Sea Fish. Res. Stn. Israel. 43: 18-54. [ Links ]
Vervoort, W. 1968. Report on a collection of Hydroida from the Caribbean region, including an annotated checklist of Caribbean hydroids. Zool. Verh. 92: 1-124.
Wallace, W.S. 1909. A collection of hydroids made at the Tortugas, during May, June and July, 1908. Carnegie Inst. Washington. 7: 136-138.
Weill, R. 1934. Contribuition à létude des cnidaires et leurs nematocysts I, II. Trav. Stn. Zool. Wimereux, 10: 1-347, 11: 347-701.
Weismann, A. 1883. Die Entstehung der Sexualzellen bei den Hydromedusen. Zugleich ein Beitrag zur Kenntnis des Baues und der Lebenserscheinungen dieser Gruppe. Jena, Gustav Fisher. 295 p.
Winge, Ö. 1923. The Sargasso Sea, its boundaries and vegetation. Report on the Danish Oceanographical Expeditions 1908-1910 to the mediterranean and Adjacent Seas. Vol. III. Misc. Papers. 2: 1-34.
Yamada, M. 1959. Hydroid fauna of Japanese sea and its adjacent waters. Pub. Akkeshi Mar. Biol. Sta. 9: 1-101. [ Links ]
1 Marine Biology and Ecology Research Group, School of Biological Sciences, University of Plymouth, Drake Circus, Plymouth, Devon, PL4 8AA, United Kingdom; F.Kelmo@plymouth.ac.uk – fkelmo@ufba.br












 uBio
uBio 

