Revista de Biología Tropical
versão On-line ISSN 0034-7744versão impressa ISSN 0034-7744
Rev. biol. trop vol.51 no.1 San José Mar. 2003
Abstract
In this study we measured the community respiration and the bacterial respiration as part of the overall degradation process of organic material. Additionally, the turnover rates of the pools of dissolved free glucose and acetate as representatives of the fraction of easily degradable low molecular organic solutes were determined. The study was performed in several coastal lagoons of the "Outer Delta of the Río Magdalena" in northern Colombia. The lagoons can be separated into two groups: The first group contains highly productive brackish lagoons with chl a concentrations ranging from 62 – 130 µg/l. The second group consists of less productive freshwater lagoons with chl a between 5.5 – 19 µg/l. Turnover rates of glucose and acetate were very fast in the highly productive lagoons resulting in turnover times of less than 20 min for both compounds. In the less productive systems the cycling of glucose and acetate was much slower. Here the mean values of the turnover times were 2 hr for glucose and 1.5 hr for acetate. The rates of bacterial DNA-formation measured as thymidine incorporation differed significantly between both groups of lagoons, being very high (1.86 – 2.76 nmol/l/hr) in the highly productive and relatively low (0.073 – 0.55 nmol/l/hr) in the less productive group. Water column community respiration ranged between 122 and 16 µg C/l/hr with means of 88 µg C/l/hr in the highly and 19 µg C/l/hr in the less productive group. In the first group the mean values of the bacterial contribution to community respiration amounted to 37% and in the second group to 18%. The bacterial respiration was determined in an indirect way via bacterial biomass production and assuming a growth efficiency of 50%. It is discussed whether this relatively high growth efficiency allows reasonable results in both groups of lagoons.
Key words: Ciénaga Grande de Santa Marta, community respiration, bacterial activity, growth efficiency, glucose turnover, tropical coastal lagoon
Cycling of organic matter in aquatic environments is based on the close interaction between processes leading to a production of material and those causing a decomposition of the produced organic substances. The organic material in aquatic systems occurs in form of living organisms, non-living particulate debris (usually called organic detritus) and dissolved substances, the latter fraction normally forming the largest part of the organic carbon (Parsons et al. 1984). The concentration of dissolved organic carbon (DOC) varies over a range of about two orders of magnitude. The lowest values occur in deep ocean waters (0.5 mg/l, Kähler and Koeve 2001), in shallow estuaries concentrations of up to 20 mg/l may be found (Bianchi et al. 1997) and DOC-concentrations of almost 50 mg/l were reported by Mann and Wetzel (1995) from a wetland pond in the southern USA. The bulk of the dissolved organic substances (the distinction between finest particles and dissolved material is difficult and somewhat arbitrary) consists of high molecular organic compounds. Only a small fraction is formed by low molecular organic solutes like dissolved free amino acids, monosaccharides and organic (carboxylic) acids. In surface waters of the equatorial Pacific, the combined amount of dissolved neutral mono-saccharides, of which glucose was the dominant compound, represented only <1% of the total DOC-concentration (Rich et al. 1996).
A significant fraction of the dissolved organic material is quite refractory against bacterial attack like e.g. humic substances. Another part, namely the high molecular compounds, has to be hydrolyzed by exo-enzymatic processes before the resulting products can be taken up by bacteria (Hoppe 1993). Only the low molecular fraction (mostly monomers) can be incorporated directly by bacteria and thus removed from the water down to very low concentrations. These so called "threshold concentrations" of different compounds like free aminoacids or monosaccharides are in the nanomolar range. Skoog et al. (1999) found between 2-15 nmol/l of glucose in surface waters of the Gulf of Mexico. Whereas regional and seasonal variations of the concentrations of easily utilizable organic substances are relatively small (Michaelis and Ittekkot 1982), high variations of their turnover rates have been reported. Thus, the dynamics of the pool of a special substance, i.e. the amount of material channelled through the pool of this compound, depends to a much higher degree on the turnover rate than on variations of concentration (Williams 1981, Gocke and Rheinheimer 1988).
Whereas the zooplankton requires particulate organic material as food source, the heterotrophic bacteria are the only important group of organisms (concerning their role in carbon dynamics) which can use particulate as well as dissolved organic matter. The bacteria therefore play a key role in the cycling of organic carbon in aquatic environments (Williams 1981). The total decomposition of particulate and dissolved organic substances can easily be determined by measuring the community respiration. This is one of the simplest and least ambiguous estimations of heterotrophic metabolic activity that can be directly related to organic matter oxidation (Biddanda et al. 1994). On the other hand, the direct determination of the bacterial respiration is a difficult task. Although microbial respiration rates are higher per unit biomass than those of larger organisms, the respiration rate per unit volume of water (especially in low productive systems) is often too small to be measured directly. In productive coastal environments, however, a fractionated filtration (i.e. the separation of bacteria from other organisms using filters of small pore size), preceding respiration measurements, might be a way to achieve this goal, but a clear separation is mostly impossible due to size overlapping of bacteria with the smallest algae and nanoflagellates. The contribution of bacteria to community respiration, therefore, has to be determined in an indirect form which can be done by first determining bacterial secondary production and then calculating bacterial respiration by assuming a certain growth efficiency. There are, however, several uncertainties. First, the determination of bacterial secondary production from incorporation rates of tritiated thymidine or leucine, requires itself the adoption of several conversion factors. Then a specified factor for growth efficiency has to be used. None of these factors is stable as each varies according to environmental conditions. Taking all these problems together, the determination of bacterial respiration remains a complicated problem.
Being well aware of the inherent problems, we nevertheless attempted to determine bacterial respiration and its contribution to community respiration. Partition of the aerobic metabolism between different physiological groups in the water column, gives a direct estimate of the importance of bacterial decomposition in the overall cycling of organic carbon and allows the amount of organic material available for non-bacterial consumers to be determined.
The specific tasks of this study, were to measure the turnover rates of low molecular organic compounds and the magnitude of bacterially mediated decomposition of organic material, in two groups of tropical coastal lagoons in northern Colombia, which are distinguished by their salinity, input of allochthonous material and magnitude of biological activity. In these lagoons, such measurements which are basic for understanding the cycling of organic matter in aquatic systems, were never done before.
Materials and methods
Study sites: Since a detailed description of the study area is given by Palacio (1983) and Botero and Mancera (1996), the dates presented here will concentrate on those geographic, hydrographic and biologic features which are important for understanding our results. The area forms part of a delta lagoon complex, the so-called "Outer Delta of the Río Magdalena" (DERM = Delta Exterior del Río Magdalena), which is located at the northern coast of Colombia (Fig. 1). This enormous wetland area is bordered on the western side by the Río Magdalena and on the eastern side by the high mountain region Sierra Nevada de Santa Marta. The northern boundary is the Caribbean Sea. The study area lies between 10° 45–11° 05 N and 74° 16–74° 46 W.
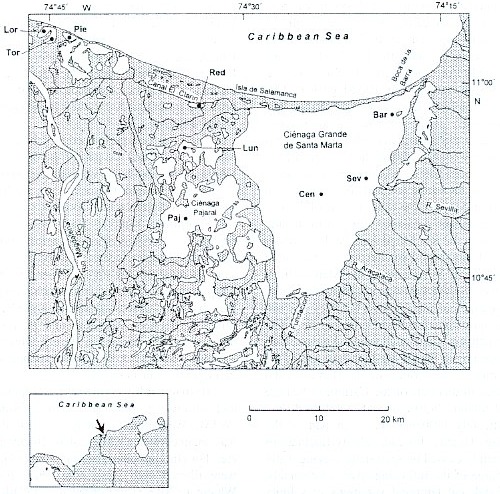
Several lagoons are lying in this wetland area, the largest one is the brackish water system Ciénaga Grande de Santa Marta, in which three stations were defined. It forms an enormous triangle (450 km2 ) bordered to the north by the Isla Salamanca, a long and narrow sand barrier. On its eastern side a fertile plane area mostly used for banana and oil palm plantations, separates the Ciénaga Grande from the Sierra Nevada de Santa Marta. On its southern and western side a huge inundation and accumulation area exists with large swamps and numerous lagoons. This socalled "Complejo Pajarales" represents a mangrove area , where Avicennia germinans, Rhizophora mangle and Laguncularia racemosa predominate. Three of our studied lagoons, the Ciénaga Pajaral, the Ciénaga La Luna and the Ciénaga La Redonda, are situated within this area. A system of channels exists connecting the lagoons with the Ciénaga Grande and the Río Magdalena. Three further sampling sites, the Ciénaga La Piedra, Ciénaga El Loro and Ciénaga El Torno are situated near the mouth of the Río Magdalena. This area is called "Complejo Isla Salamanca Occidental". It is influenced by the Río Magdalena via an artificial channel. The area is not directly connected with the Ciénaga Grande or the lagoons within the Complejo Pajarales. Since the whole wetland area is situated in a semi-arid region, the water regime of the system depends on a balance between freshwater input from the Río Magdalena (and to a lesser degree from several smaller rivers originating in the Sierra Nevada de Santa Marta which, however, control significantly the hydrographic dynamics of the Ciénaga Grande) and salt water intrusion from the Caribbean Sea. The latter happens via a small (about 120 m) artificial opening at the far north-eastern end of the C. Grande, the "Boca de la Barra". Tidal changes in this part of the Caribbean Sea are small (about 20-30 cm) and therefore do not significantly influence the main part of the Ciénaga Grande. Instead, salinity fluctuations are mainly caused by seasonally varying water discharge of the inflowing rivers or channels. Regional and temporal variations of salinity are between 0–40 PSU (Hernández 1986).
The whole study area is subjected to a series of environmental stress factors, the most important ones are associated with alterations of the freshwater regime (Mancera and Vidal 1994, Botero and Salzwedel 1999). These lead to soil hypersalinization causing a mass mortality of more than 70% of the original mangrove forest coverage (510 km2 ) during the past 40 years (Botero and Mancera 1996).
All lagoons within the Outer Delta of the Río Magdalena, including the enormous Ciénaga Grande, are extremely shallow. Depths are generally between 1.5 and 1.8 m. The primary productivity of the C. Grande is very high (Hernández and Gocke 1990). The trophic status of the other coastal lagoons is less known, since detailed measurements have not been performed yet. A study made by Rondón (1990) in the Complejo Pajarales, however, suggests a high productivity of C. Pajaral and C. La Luna and a lower one of C. La Redonda. The productivity of the lagoons of the Complejo Isla Salamanca Occidental is probably quite low due to their high inorganic water turbidity.
The study was performed in February and March 1997, which are part of the dry season. During these months the water discharge of the Río Magdalena and the rivers from the Sierra Nevada usually reaches its lowest level.
Sampling: Samples were taken with a Niskin sampler directly below the surface. Since this sampler has a length of 50 cm the samples represent the upper half-meter of the water column. Samples from each group of lagoons were processed simultaneously, the time lap between sampling and processing never exceeded one hour, during which the samples were kept at their original temperature in a light tight insulation box.
Hydrographic features: Temperature and salinity were measured with a TS-probe (WTW, Weilheim, Germany). Secchi depth was obtained with a white disk of 20 cm diameter. For chlorophyll a determinations samples were filtered onto glass fibre filters (GF/F, Whatman). These were stored at –20°C till further processing which was done about two months later. Pigments were extracted with 90% acetone and measured by HPLC.
Turnover rates (Tr ) of glucose and acetate: To determine the turnover rates of dissolved glucose and acetate, 0.04 µCi of uniformly labeled 14 C-glucose and 14 C-acetate respectively were added to sets of 4 small plastic vials containing 20 ml of sample. One vial of each set served as control by adding 200 µl of 37% formaldehyde. Samples were then incubated for 20 minutes at ambient temperature in a light tight insulation box (similar to the TTI measurements, see below). Aliquots of 3 or 5 ml were filtered onto 0.2 µm cellulose-acetate membrane filters and rinsed three times with 5 ml of prefiltered water from the respective station. This procedure allows only the net uptake to be measured. To calculate gross uptake, a percentage respiration of 25% with glucose and 35% with acetate was assumed (Gocke 1977).
Thymidine incorporation (TTI): To triplicate 3 ml portions of the samples, 5 µCi of 3 H-thymidine (SA = 64 Ci/mmol, final concentration 26 nmol/l) were added. After an incubation of 20 minutes at their original temperature in an insulation box the bacterial activity was arrested by adding 100 µl of 37% formaldehyde. Samples poisoned with formaldehyde prior to the radioisotope additions served as controls. Samples were then brought to the laboratory , where 1 ml (from the stations of the Ciénaga Grande and the lagoons of the Complejo Pajarales) or 2 ml aliquots (from the Isla Salamanca complex) were filtered onto 0.2 µm polycarbonate filters and rinsed 10 times with 1 ml of ice-cold 5% trichloroacetic acid. It was necessary to filter such small volumes since the filters were rapidly blocked by high seston concentrations. The retained radioactivity was determined with a scintillation counter in Germany. The method applied is similar to that one introduced by Fuhrman and Azam (1982). For estimations of bacterial biomass production (µg C/l/hr) conversion factors of 1.1 x 109 cells per nmol of incorporated thymidine, a mean bacterial cell volume of 0.05 µm3 and 0.25 x 10-6 µg C per µm3 of bacterial volume were employed (Riemann et al. 1987). Knowing the bacterial biomass production, the bacterial degradation of organic material can be estimated assuming a metabolic efficiency of 50% (Ducklow 1983).
(Unfortunately the samples for estimating bacterial numbers were lost during their transport to Germany).
Community respiration (CR): Sets of 4 glass flasks of 50 ml (nominal volume) were cautiously filled with the sample. Two of them served to determine the initial oxygen concentration, the remaining two were incubated for 6 hours at or near their original temperature in a light tight insulation box. Oxygen measurements were made using the Winkler technique. A respiration quotient (RQ) of 0.83 was employed to convert O2 -units into µg of organic carbon respired by the suspended organisms (Vollenweider 1974).
Results
Hydrographic features: The hydrographic features are presented in Table 1. Water temperature ranged from 24.3°C (C. La Piedra) to 30.8°C (C.Grande at St. Boca R. Sevilla). Generally the temperature in the lagoons, which were more affected by river water, was several degrees lower than in the C. Grande and C. Pajaral. Salinity values were between 0.1 (pure river water) and 21.8 PSU (C. Grande at St. Barra). Secchi depths were very small in all studied lagoons ranging between 20 cm (C. La Redonda) and 30 cm (C. Grande, St. Barra) thus indicating an extremely high turbidity. The saturation of the water at 0.5 m depth with dissolved oxygen ranged between 79% (C. La Luna) and 121% in the Ciénaga Grande at St. Barra. Since this parameter underlies significant daily fluctuations, it should be mentioned that the data were obtained between 8:40 and 10:25, thus they are not directly comparable. Time course fluctuations of O2 -saturation (not shown here) at St. Barra in the C. Grande as well as in C. Pajaral and C. El Loro showed that in the latter lagoon the saturation oscillated around 100% between 10:25 and 13:40. In the two other lagoons the oxygen content reached extreme supersaturations at the end of the observation period (at about 13:30).
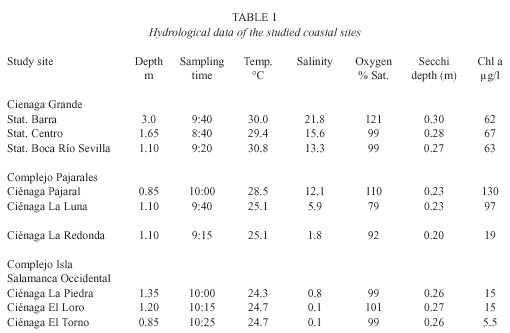
Biological features: The chlorophyll a concentrations were relatively low in the "limnic" lagoons (between 5.5 and 19 µg/l) and very high in the brackish water systems (62–130 µg/l).
The turnover rates (T r ) of dissolved glucose and acetate ranged between 0.26 hr -1 for glucose in C. El Torno and >3 hr -1 for glucose as well as acetate in the more productive brackish lagoons (Fig. 2). Expressed as time units, the longest turnover times observed were 3.8 hr for the pool of dissolved glucose and 3.3 hr for acetate in C. El Torno, while in the brackish lagoons the turnover times for both compounds were less than 20 min. Probably the Tr of both low molecular compounds do not differ significantly as inferred from Fig. 2.
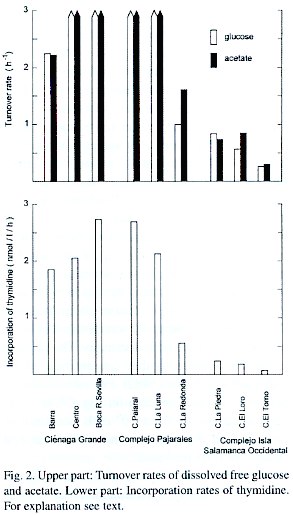
The incorporation rates of thymidine as a measure of bacterial activity were very elevated in the highly productive brackish lagoons and relatively low in the freshwater lagoons (Fig. 2). Thus, the resulting pattern resembled very much the picture already obtained with the glucose and acetate turnover rates. When the TTI values were converted to bacterial Fig. 2. Upper part: Turnover rates of dissolved free glucose and acetate. Lower part: Incorporation rates of thymidine. For explanation see text. carbon produced per liter and hour, i.e. to bacterial secondary production (BSP), the highest production rates were found in Ciénaga Grande at St. Boca R. Sevilla and in C. Pajaral. Here the BSP amounted to 37.5 and 37.3 µg C/l/hr respectively. In the less productive limnic lagoons bacterial secondary production rates were much lower. In C. El Torno it reached only 1.0 µg C/l/hr. Assuming a metabolic efficiency of 50% which means that half of the organic material taken up by the heterotrophic microorganisms is respired for energy requirement and the other half is converted to bacterial biomass, then bacterial respiration is numerically equal to bacterial secondary production. Thus, for example, in the C. Pajaral 37.3 µg of organic carbon is degraded per liter and hour, whereas in C. El Torno it is only 1.0 µg C/l/hr. The reproducibility of the measurements with 14C and 3H-compounds was very good (mean standard deviation of the 3 replicas <3%). The radioactivity of the blanks amounted to about 1% (highly productive lagoons) respectively <5% (less productive lagoons) of the corresponding samples.
The community respiration (CR) ranged between 122 µg C/l/hr (C. Pajaral) and 16 µg C /l/hr in C. El Torno. As with respect to bacterial activity the CR in the highly productive brackish lagoons was much higher than in the less productive freshwater lagoons (Fig. 3). The relative difference, however, between the highest and lowest community respiration rates was significantly smaller than with bacterial activity. Thus, the mean value of CR was 88 µg C/l/hr in the 5 brackish lagoons and 19 µg C/l/hr in the freshwater ones, which gives a ratio of 4.6:1. On the other hand, the ratio with regard to thymidine incorporation between both groups of lagoons was 8.8:1.
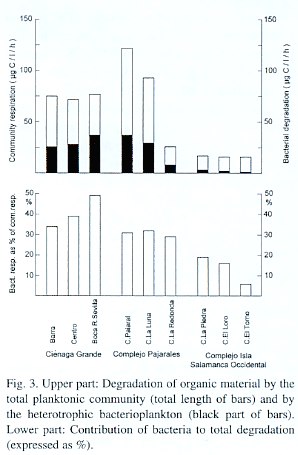
The contribution of heterotrophic microorganisms to the degradation of organic material depended very much on the trophic status of the lagoons. It ranged between 49% (C.Grande at St. Boca R. Sevilla) and 6% (C. El Torno). The mean values were 37% (highly productive lagoons) and 18% (less productive lagoons). With respect to community respiration as well as bacterial contribution to CR the differences between both groups of lagoons were highly significant (<0.01).
Discussion
At first glance, the geographic position of the nine lagoons or stations of the study site (Fig. 1) would suggest a separation arrangement into three different groups: 1) the three stations of the Ciénaga Grande, 2) the lagoons of the Complejo Pajarales and 3) the remaining three lagoons of the Complejo Isla Salamanca Occidental. A closer look, however, makes a differentiation of only two groups better suitable: The first group would then consist of the C. Grande with its three stations together with the Ciénaga Pajaral. These lagoons are brackish water systems with high biological activity as revealed by their high chlorophyll a concentrations. The turbidity of the water is high, caused mainly by plankton and organic detritus Fig. 3. Upper part: Degradation of organic material by the total planktonic community (total length of bars) and by the heterotrophic bacterioplankton (black part of bars). Lower part: Contribution of bacteria to total degradation (expressed as %). resulting in a greenish water color. The second clearly distinguishable group contains the lagoons of the Complejo Isla Salamanca Occidental and the Ciénaga La Redonda of the Complejo Pajarales. These "limnic" coastal lagoons are pure or almost pure freshwater systems, which are also very turbid. This turbidity, however, is due to high amounts of fine clay particles originating from the Río Magdalena and introduced into the lagoons via a system of channels. It gives the lagoons a yellow-brownish water color. The chl a concentrations are relatively low. The remaining lagoon, the Ciénaga La Luna, occupies a transition stage between both groups of lagoons having a reduced salinity and an olivegreen water color. This is due to its position between the strongly riverine influenced lagoons and the brackish ones. Since, however, its chl a concentration is high, the C. La Luna resembles from a biological viewpoint more the typical brackish water lagoons of the study area and is thus placed in this group.
In the highly productive brackish lagoons of the study area the turnover rates of glucose and acetate were very high leading to turnover times of less than 20 min, but the exact turnover rates and times cannot be calculated. This is due to the fact that the incubation time applied, proved to be too long (it should be much shorter than the turnover time). Adjustments of the method were not possible, because preliminary studies could not be made at the study sites, since it was impossible to count the radioactivity before the end of the whole study. It is thus probable that the turnover times of glucose and acetate in the highly productive lagoons were well below 20 min. To sustain such short turnover times, a very close coupling between those processes which cause a replenishment, on the one side, and a depletion of the respective pools, on the other side, must exist. Replenishment processes are exudation by phytoplankton, sloppy feeding and excretion by zooplankton, hydrolysis of high molecular compounds by bacteria (Lee and Henrichs 1993). The most important depletion process is the incorporation of the substances by bacteria. The high density of phytoplankton indicated by the elevated concentration of chlorophyll a (Table 1) and the high primary production (Hernández and Gocke 1990, Gocke et al. in prep.) favour the replenishment processes, whereas the extremely high abundance of bacteria (maximal values up to 98 x 106 cells/ml, Gocke and Hernández unpubl.) resulting in an elevated bacterial activity (Fig. 2) is responsible for the high turnover rates. Water temperatures of around 30°C accelerate the above mentioned processes.
Turnover times of less than 20 min in the water column of natural aquatic systems have not yet been reported in the literature. Gocke and Rheinheimer (1988) studied the turnover times of glucose and acetate as well as aspartic acid in the anthropogenically influenced lower Elbe River. The shortest turnover times they found were observed during summer (22°C) and amounted to 53 min for glucose and 43 min for acetate. The flux rates of these compounds were not computed because their concentrations could not be measured during the present study. Rich et al. (1996) found that glucose alone supported 15-47% of the bacterial production in the equatorial Pacific. In the Gulf of Mexico glucose uptake accounted for 5-15% of the bacterial production (Skoog et al. 1999). The latter authors state that the measurement of free glucose assimilation includes the uptake of combined glucose too, which indicates that the dissolved monosaccharides may be supplied very fast from the hydrolysis of polymers. This may be an important pathway in our study area, since in the mangrove bordered lagoons and channels of the Outer Río Magdalena Delta leaching of mangrove litter may provide large quantities of monosaccharide containing polymers (Benner et al. 1988, Mueller and Ayukai 1998).
Gocke and Rheinheimer (1988) found in the lower Elbe River that the differences between the turnover times of glucose, aspartic acid and acetate, which belong to three different classes of substances, were relatively small (maximum difference less than a factor of three). They concluded that probably the majority of free dissolved sugars, amino acids and substances occurring in the Krebs cycle might be turned over with roughly the same velocity. Our present results show that this obviously is true in tropical coastal lagoons too. This is in contrast to the observations of Rich et al. (1996) who deduced that, at least in the equatorial Pacific, the concentrations of monosaccharides other than glucose were lower and probably also their turnover rates. Our results suggest that high productive coastal lagoons with high dynamics of the pools of low molecular organic solutes obviously behave in a different way than open sea systems with respect to dissolved labile organic substances.
In the less productive freshwater lagoons the turnover rates of glucose and acetate were much lower than in the highly productive systems. In the microbiologically most active one of the less productive lagoons, the C. La Redonda, Tr of 1.6 hr -1 for acetate and 1.0 hr -1 for glucose (equal to turnover times of 37 min and 1 hr respectively) were observed. In the least productive Ciénaga El Torno the turnover times increased to 3 hr 50 (glucose) and 3 hr 20 (acetate). These turnover times seem to be relatively long, at least when compared to the productive lagoons of the study area, but nevertheless they are still very short in comparison to the nearby coastal areas of the Caribbean Sea. In the small Nenguange Bay about 80 km north-east of the Complejo Isla Salamanca Occidental (chl a concentration 0.29 µg/l) turnover times for glucose and acetate of 125 hr and 388 hr respectively were observed (Gocke et al. unpubl.). In the upper layer of the Gulf of Mexico turnover times for glucose between 60-2 400 hr were encountered by Skoog et al. (1999).
In accordance to the fast cycling of easily degradable organic compounds, the bacterial activity in the highly productive lagoons of the study area was extremely high (Fig. 2). The mean value for thymidine incorporation was 2.3 ± 0.40 nmol/l/hr. The mean for TTI in the less productive lagoons were 0.26 ± 0.21 nmol/l/hr. It might be interesting to compare the bacterial activity (and abundance) of the group of highly productive coastal lagoons in tropical Colombia with the results obtained in the Schlei Fjord, a hyperproductive brackish water system in temperate Northern Germany. In the Schlei Fjord the summer mean for TTI (only those months with water temperatures above 15°C were taken into consideration) amounted to 0.27 nmol/l/hr. The maximum incorporation rates were observed in September (water temperature 19.0 °C), when thymidine incorporation was 0.56 nmol/l/hr. Since unfortunately the samples for total bacterial numbers (TBN) in the Colombian lagoons were lost, only a very rough estimation of the bacterial counts in these lagoons can be given. Hoppe et al. (1983) found a TBN of 20.3 x 10 6 cells/ml at the center of the Ciénaga Grande and 30 x 106 cells/ml near our St. Barra. These data refer to November, when due to heavy freshwater input the salinity had decreased to an unusually low value of 0.2 PSU throughout the Ciénaga Grande. Gocke and Hernández (unpubl.) observed bacterial cell numbers around 50 x 106 /ml at Stns. Centro and Boca R. Sevilla. Their observations were made in February 1987, when the salinity was near 25 PSU. Bacterial numbers in the Schlei Fjord amounted to about 30 x 106 cells/ml during summer months. Hence, bacterial numbers in the productive coastal lagoons in Colombia are probably somewhat, but not excessively, higher than in the Schlei Fjord. In contrast to this, from the data mentioned above it becomes obvious that the bacterial activity in the highly productive Colombian lagoons is very much higher than in the Schlei Fjord, since even the maximum TTI values in the Schlei are surpassed at least by a factor of four. This is, however, largely due to temperature effects, since the water in the tropical lagoons was about 10°C warmer, which causes a temperature induced doubling or even tripling of bacterial activity (Parsons et al. 1984).
The community respiration rates differed significantly between both groups of coastal lagoons. At the three stations of the Ciénaga Grande, where bacterial activity (Fig. 2) and phytoplankton density (as revealed by chl a concentration, Table 1) were relatively uniform, almost the same CR was found (72- 77 µgC/l/hr). In the two remaining highly productive lagoons, the C. Pajaral and the C. La Luna, the CR were even greater (122 and 93 µg C/l/hr) accompanied by higher chl a concentrations but not by higher bacterial activities. In the less productive freshwater lagoons the CR were much lower (Fig. 3) and amounted to only 16-26 µg C/l/hr. The CR data at St. Centro in the Ciénaga Grande are within the range reported by Hernández and Gocke (1990), who observed in February and March 1987 CR rates of 101 and 36 µg C/l/hr respectively. In a salt marsh estuary (Portugal) Cunha et al. (1999) found CR rates of 4.4 µg C/l/hr in the marine part and 18.8 µg C/l/hr in the more eutrophic brackish part of the system. In the hypertrophic inner part of the Schlei Fjord (Northern Germany) annual means of community respiration amounted to 12.6 µg C/l/hr. The maximum summer value was 26.9 µg C/l/hr. In the coastal region near the mouth of the Schlei Fjord the CR rates were 3.1 and 6.4 µg C/l/hr respectively (Gocke unpubl.). Biddanda et al. (1994) observed CR rates between 0.5-4.5 µg C/l/hr in the less productive slope and the highly productive shelf waters of the Gulf of Mexico. Our results of the present study demonstrate that the overall degradation and mineralization processes in the tropical coastal lagoons of the study site take place at a very high velocity. Even the CR in the less productive lagoons equals or surpasses those of polyproductive systems in temperate regions.
Comparing community respiration as a measure of total decomposition of organic material with the decomposition mediated by bacteria we found that in the productive lagoons between one third to half of the organic material was decomposed by bacteria. In the less productive lagoons bacteria were responsible for only about one fifth of the total decomposition. Ciénaga La Redonda does not fit so well into this scheme, since here the decomposition due to bacteria amounted to 26% of the total. On the other side, the contribution of bacteria to overall respiration amounted to only 6% in Ciénaga El Torno. In Louisiana shelf waters Biddanda et al. (1994) found a bacterial contribution to CR of 49%. Morales-Zamorano et al. (1991) reported that in a tidal channel of a coastal lagoon in Baja California >50% of the respiratory activity was in the <0.8 µm fraction of the plankton, which most probably are almost exclusively bacteria.
The bacterial contribution to community respiration determined by us in the coastal lagoons of northern Colombia are at the lower end of the reported data, but nevertheless they seem to be within reasonable limits. Obviously the magnitude of the community respiration sets the upper limit for bacterial respiration alone. One could imagine natural aquatic biotopes, where environmental conditions are so unfavorable that only specialized heterotrophic bacteria could survive, which then must depend on the input of allochthonous organic material. If such biotopes exist at all, there can be only few and small ones which must be transitory in nature. The other extreme, i.e. biotopes in which autotrophic organisms (chemo- and photoautotrophic bacteria or green plants) and consumers (zoo-plankton or larger animals) exist but which is free of bacteria, is hardly imaginable. Thus, the limits for bacterial contribution to community respiration in "normal" aquatic biotopes with phytoplankton, zooplankton (and larger consumers) and bacteria will never cover the whole range between 0 and 100%, but are probably within a significantly smaller range. The range covered by our results was between 6% in the less productive Ciénaga El Torno and 49% in the highly productive Ciénaga Grande at St. Boca R. Sevilla. Our results were obtained by assuming that the bacterial growth efficiency (BGE) amounts to 50%, meaning that bacterial respiration and bacterial production are equal in size. It might be interesting to see to what extent the adoption of a different BGE influences the results of bacterial respiration and contribution to community respiration. The following considerations concerning the BGE are based on the premise that the factors used for the calculation of bacterial secondary production are reasonable (see METHODS). The factors reported in the literature differ within certain limits. We chose the factors given by Riemann et al. (1987), since they were derived from experiments with bacterial populations in eutrophic areas.
Earlier studies (Gocke 1976) have shown that bacteria, when supplied with labeled sub-stances like dissolved free amino acids, carbohydrates or organic acids, react with a BGE between 90% (leucine, lysine) and 50% (aspartic acid, malic acid). When an optimal mixture of substrates was added, bacteria grew with an efficiency of 80% (Payne and Wiebe 1978). In later studies with natural substrate mixtures, however, mostly much lower bacterial growth efficiencies were found, which ranged from a few percent up to about 60%. Generally the values fell into the range from 20 to 40% (Biddanda et al. 1994, Cole and Pace 1995, Weiß and Simon 1999). If the bacteria had a growth efficiency smaller than 50% they would require a larger amount of organic substances to maintain the measured bacterial production and hence would be responsible for a greater flux of organic material. A BGE of 25% would mean that the amount of organic material respired by the bacterial assemblage would have to be three times as large as compared to a BGE of 50% in order to maintain the same bacterial production. In that case the bacterial respiration in the Ciénaga Grande would surpass the CR which is obviously impossible. In the lagoons of the Complejo Pajarales the contribution of bacteria to overall CR would be around 90%, which seems unlikely, since the CR was high significantly correlated to the chlorophyll a concentration (Gocke et al. In prep.), thus indicating that at least a considerable part of the community respiration is due to the dark respiration of phytoplankton. Only in the lagoons of the Complejo Isla Salamanca Occidental the contribution of bacteria would represent a reasonable fraction of CR ranging from 18-57%. Obviously the bacterial growth efficiency at least in the Ciénaga Grande and the lagoons of the Complejo Pajarales must be higher than 25% and should probably be near 50% to give reasonable results.
There are some indications that the BGE is higher under special circumstances. Benner et al. (1995) concluded in their work on the Amazon River and several of its tributaries that BGE was higher during high water periods. The authors attributed this to an increased bioavailability of substrates washed into the river from inundated flood plains. Also in a eutrophied temperate lake the BGE was positively correlated to increased labile DOC (Middelboe and Sondergaard 1993). Kroer (1993) observed in batch cultures with natural bacterial assemblages that BGE increased with rising ammonium concentrations. These few literature citations suggest already that the BGE might be higher in aquatic systems with a good supply of labile organic matter having a favorable C:N:P ratio or with less favorable labile organic material accompanied with sufficient inorganic nutrients. Both prerequisites are probably fulfilled in the highly productive lagoons of our study area. In these lagoons assuming a BGE of 50% results in a bacterial respiration which is probably relatively close to the real value, whereas assuming the same BGE in the less productive lagoons might underestimate to some extent the bacterial respiration. If the BGE in the latter lagoons were around 35% the bacterial respiration would increase to values between 12 and 38% of the community respiration, which would be in an acceptable range.
As a conclusion it might be stated that the elevated magnitude of primary productivity and bacterial activity in the highly productive lagoons of the study area in conjunction with an elevated water temperature lead to extremely high turnover rates of low molecular organic substances like dissolved glucose and acetate. Although in the less productive fresh-water lagoons the turnover rates of these compounds were significantly lower, they were still almost as high as in hyperproductive aquatic systems of the temperate regions. In the highly productive brackish water lagoons. about one third of the total degradation of organic matter was due to bacterial activity whereas in the less productive freshwater lagoons the bacteria only accounted for about one fifth of the overall degradation. This contribution in the less productive systems seems to be relatively small. It could possibly be explained by the fact that the conversion factors used to calculate the bacterial secondary production and also the value taken for bacterial growth efficiency are not adequate for this kind of aquatic systems. Taken together this could mean that the bacteria play an equal or even more important role in the less productive freshwater lagoons as in the highly productive brackish water lagoons of northern Colombia.
Acknowledgements
This study was supported by a grant to the First author from a bilateral scientific interchange program between COLCIENCIAS (Colombia) and the DAAD (German Academic Exchange Service). The field trips were financed by the Instituto de Investigaciones Marinas y Costeras (INVEMAR) in Santa Marta, Colombia. We thank the director, the scientific and technical board of the INVEMAR for constant encouragement during the study. The students Adriana Garavito and Liliana González are gratefully acknowledged for field and laboratory assistence. Last, but not least, we are specially indebted to Mr. Martin Montaño for his most skillfull aid as boat helmsman during the field trips. Finally we like to thank the anonymous reviewers for their valuable and constructive criticism.
Resumen
Este estudio midió la respiración de la comunidad planctónica y la respiración bacteriana como parte del proceso de degradación del material orgánico. Adicionalmente se determinaron las tasas de renovación de los acervos de glucosa libre disuelta y acetato, como representativas de la fracción de compuestos orgánicos de bajo peso molecular facilmente degradables. El estudio fue desarrollado en varias lagunas costeras del Delta Exterior del Río Magdalena, al norte de Colombia. Las lagunas pueden ser divididas en dos grupos. El primero incluye lagunas salobres altamente productivas, con concentraciones de clorofila a entre 62-130 µg/l. El segundo grupo está conformado por lagunas de agua dulce menos productivas con clorofila a entre 5.5-19 µg/l. Las tasas de renovación de glucosa y acetato fueron muy altas en las lagunas muy productivas, con tiempos de renovación menores de 20 minutos para ambos compuestos. En los sistemas menos productivos los ciclos de ambas sustancias fueron mas lentos. Aquí los valores promedio de los tiempos de recambio fueron de 2 hr para glucosa y de 1.5 hr para acetato. Las tasas de formación de ADN, medida como incorporación de timidina, fueron significativamente diferentes entre ambos grupos de lagunas, siendo muy alta (1.86 – 2.76 nmol/l/hr) en el grupo altamente productivo y relativamente baja (0.073 – 0.55 nmol/l/hr) en el menos productivo. La respiración de la comunidad planctonica en la columna de agua estuvo entre 122 y 16 µg C/l/hr, con promedios de 88 µg C/l/hr en el grupo altamente productivo y 19 µg C/l/hr en el menos productivo. En el primer grupo los valores promedio de la contribución bacteriana a la respiración de la comunidad planctónica alcanzaron 37% y en el segundo grupo 18%. La respiración bacteriana fue determinada indirectamente a través de la producción bacteriana, asumiendo una eficiencia en el crecimiento de 50%. Se discute si esta eficiencia relativamente alta en el crecimiento lleva a resultados razonables en ambos grupos de lagunas.
References
Benner, R., R.E. Hodson & D. Kirchman. 1988. Bacterial abundance and production on mangrove leaves during initial stages of leaching and biodegradation. In T.E. Cappenberg & C.L.M. Steenbergen (eds.). Proc. of the Third Int. Workshop on the Measurement of Microbial Activities in Aquatic Ecosystems 31: 19-26.
Benner, R., S. Opsahl, G. Chin-Leo, J.E. Richey & B.R. Forsberg. 1995. Bacterial carbon metabolism in the Amazon River system. Limnol. Oceanogr. 40: 1262-1270. [ Links ]
Bianchi, T.S., M. Baskaran, J. DeLord & M. Ravichandran. 1997. Carbon cycling in a shallow turbid estuary of southeast Texas: The use of plant pigment biomarkers and water quality parameters. Estuaries 20: 404-415. [ Links ]
Biddanda, B., S. Opsahl & R. Benner. 1994. Plankton respiration and carbon flux through bacterioplankton on the Louisiana Shelf. Limnol. Oceanogr. 39:1259-1275. [ Links ]
Botero, L. & J.E. Mancera. 1996. Síntesis de los cambios de origen antrópico ocurridos en los últimos 40 años en la Ciénaga Grande de Santa Marta (Colombia). Rev. Acad. Colom. Cienc. 20(78): 465-474. [ Links ]
Botero, L. & H. Salzwedel. 1999. Rehabilitation of the Ciénaga Grande de Santa Marta, a mangrove estuarine system in the Caribbean coast of Colombia. Ocean Coast. Manage. 42: 243-256.
Cole, J.J. & M.L. Pace. 1995. Bacterial secondary production in oxic and anoxic freshwaters. Limnol. Oceanogr. 40: 1019-1027. [ Links ]
Cunha, M.A., M.A. Almeida & F. Alcântara. 1999. Compartments of oxygen consumption in a tidal mesotrophic estuary (Ria de Aveiro, Portugal). Acta Oceanol. 20: 227-235.
Ducklow, H.W. 1983. Production and fate of bacteria in the oceans. BioScience 33: 494-499. [ Links ]
Fuhrman, J.A. & F. Azam. 1982. Thymidine incorporation as a measure of heterotrophic bacterioplankton production in marine surface waters: evaluation and field results. Mar. Biol. 66: 109–120. [ Links ]
Gocke, K. 1976. Respiration von gelösten organischen Verbindungen durch natürliche Mikroorganismen-Populationen. Ein Vergleich zwischen verschiedenen Biotopen. Mar. Biol. 35: 375-383.
Gocke, K. 1977. Comparison of methods for determining the turnover times of dissolved organic compounds. Mar. Biol. 42: 131–141. [ Links ]
Gocke, K. & R. Rheinheimer. 1988. Microbial investigations in rivers. VII. Seasonal variations of bacterial numbers and activity in eutrophied rivers in Northern Germany. Arch. Hydrobiol. 112: 197-219. [ Links ]
Hernández, C. 1986. Producción primaria y dinámica del fitoplancton en la Ciénaga Grande de Santa Marta, Colombia. Tesis de Maestría, Universidad Nacional de Colombia, Bogotá, Colombia, 177 p.
Hernández, C. & K. Gocke. 1990. Productividad primaria en la Ciénaga Grande de Santa Marta, Colombia. An. Inst. Invest. Mar. Punta de Betín 19–20: 101–119. [ Links ]
Hoppe, H.-G. 1993. Use of fluorigenic model substrates for extracellular enzyme activity (EEA) measurements of bacteria. pp. 423-431. In P. F. Ke mp, B.F. Sherr, E.B. Sherr & J.J. Cole (eds.). Handbook of methods in aquatic microbial ecology. Lewis, Boca Raton, California, USA.
Hoppe, H.-G., K. Gocke, D. Zamorano & R. Zimmermann. 1983. Degradation of macromolecular organic compounds in a tropical lagoon (Ciénaga Grande, Colombia) and its ecological significance. Int. Rev. Ges. Hydrobiol. 68: 811-824. [ Links ]
Kähler, P. & W. Koeve. 2001. Marine dissolved organic matter: can its C:N ratio explain carbon overcon-sumption? Deep-Sea Res. 48: 49-62. [ Links ]
Kroer, N. 1993. Bacterial growth efficiency on natural dissolved organic matter. Limnol. Oceanogr. 38: 1282-1290. [ Links ]
Lee, C. & S.M. Henrichs. 1993. How the nature of dissolved organic matter might affect the analysis of dissolved organic carbon. Mar. Chem. 41: 105-120. [ Links ]
Mancera, J.E. & A. Vidal. 1994. Florescimiento de microalgas relacionado con la muerte masiva de peces en el complejo lagunar Ciénaga Grande de Santa Marta, Caribe Colombiano. An. Inst. Invest. Mar. Punta de Betín 23: 103-117. [ Links ]
Mann, C.J. & R.G. Wetzel. 1995. Dissolved organic carbon and its utilization in a riverine wetland ecosystem. Biogeochem. 31: 99-120. [ Links ]
Michaelis, W. & V. Ittekkot. 1982. Biogeochemistry of rivers: field and analytical techniques. Mitt. Geol.-Paläont. Inst. Univ. Hamburg, SCOPE/UNEP Sonderb. 52: 69-89. [ Links ]
Middelboe, M. & M. Sondergaard. 1993. Bacterioplankton growth yield: Seasonal variations and coupling to substrate lability and beta-glucosidase activity. Appl. Environ. Microbiol. 59: 3916-3921. [ Links ]
Morales-Zamorano, L.A., R. Cajal-Medrano, E. Orellana-Cepeda & L. C. Jimenez-Perez. 1991. Effect of tidal dynamics on a planktonic community in a coastal lagoon of Baja California, Mexico. Mar. Ecol. Progr. Ser. 78: 229-239. [ Links ]
Mueller, H. & T. Ayukai. 1998. Concentration and molecular weight distribution of dissolved organic carbon in a mangrove creek in the Hinchinbrook area, Australia. Mangroves Salt Marshes 2: 231-235. [ Links ]
Palacio, J.A. 1983. Die benthische Makroinverte-bratenfauna der tropischen Ästuarregion Ciénaga Grande de Santa Marta (Kolumbien) und ihre Aktivität im Wechsel zwischen Trocken- und Regenzeit. Tesis de Doctorado, Ruhr-Universität Bochum, Germany. 248 p.
Parsons, T.R., M. Takahashi & B. Hargrave. 1984. Biologic oceanographic processes. Pergamon, London, 330 p. [ Links ]
Payne, W.J. & W.J. Wiebe. 1978. Growth yield and efficiency of chemosynthetic microorganisms. Annu. Rev. Microbiol. 32: 155-183. [ Links ]
Riemann, B., P.K. Bjørnsen, S. Newell & R. Fallon. 1987. Calculation of cell production of coastal bacteria based on measured incorporation of 3 H-thymidine. Limnol. Oceanogr. 32: 471-476. [ Links ]
Rich, J.H., H.W. Ducklow & D.L. Kirchman. 1996. Concentration and uptake of neutral monosaccharides along 140 degree W in the Equatorial Pacific: Contribution of glucose to heterotrophic bacterial activity and the DOM flux. Limnol. Oceanogr. 41:595-604.
Rondón, E.H. 1990. Estimación de la productividad fitoplanctónica en ciénagas del complejo Pajarales, Caribe Colombiano. Tesis Biología, Universidad Javeriana, Bogotá, Colombia. 80 p.
Skoog, A., B. Biddanda & R. Benner. 1999. Bacterial utilization of dissolved glucose in the upper water column of the Gulf of Mexico. Limnol. Oceanogr. 44:1625-1633. [ Links ]
Vollenweider, R.A. 1974. Methods for measuring production rates, pp. 51-150. In R.A. Vollenweider (ed.). Primary production in aquatic environments. IBP Handbook No. 12, Blackwell Sci., Oxford, London. [ Links ]
Weiß, M. & M. Simon. 1999. Consumption of labile dissolved organic matter by limnetic bacterioplankton: The relative significance of amino acids and carbohydrates. Aquat. Microb. Ecol. 17: 1-12.
Williams, P. & J. LeB. 1981. Incorporation of microheterotrophic processes into the classical paradigm of the planktonic food web. Kieler Meeresforsch. Sonderb. 5: 1-28.
1 Institut für Meereskunde, 24105 Kiel, Germany. Fax: +49-431-6001515, e-mail: kgocke@ifm.uni-kiel.de
2 Department of Biology, University of Louisiana at Lafayette. P.O.Box 41297 Lafayette, LA 70504 USA
3 Universidad de Cordoba, Colombia












 uBio
uBio 

