Revista de Biología Tropical
versão On-line ISSN 0034-7744versão impressa ISSN 0034-7744
Rev. biol. trop vol.48 no.2-3 San José Jun. 2000
forest herb Begonia decandra (Begoniaceae)
Roberto A. Cordero S.1
Received 28-II-1999. Corrected 18-II-2000. Accepted 8-III-2000.
Abstract
The effect of two natural light-growing conditions (understory versus light gaps) and the interaction with nutrient availability (through fertilization) was studied in the understory herb Begonia decandra, in the Luquillo Experimental Forest in Puerto Rico. Sixteen potted plants obtained from cuttings were randomly chosen and distributed in each of eighth forest environments (four light gaps and four understories), for a total of 128 plants. Fertilizer was applied to half of the plants in each site. After seven months in the two given microenvironments, increased light and fertilization resulted in greater growth and some changes in the biomass allocation patterns. All measured variables responded similarly to reported changes for tree seedlings and saplings from other tropical and subtropical regions. Total growth parameters (height, biomass and leaf area) were very sensitive to increases in the main resource (light). The addition of nutrients treatments were less important in producing changes in the allocation variables (root to shoot ratio, leaf area ratio, and specific leaf mass) under conditions of high light availability. Changes due to nutrient levels were relatively greater on plants grown under understory conditions. Also, small light differences among sites can cause significant changes in the variables related to total growth. Lastly, plant mortality in the nutrient treatments was found to be independent of mortality in two forest light environments. Some hypotheses about resource acquisition and plant growth are not supported by this data.
Key words
Begonia, biomass allocation, canopy gaps, Puerto Rico, plant mortality, understory herb.
It has been established, in general, that shade-tolerant understory plants from tropical forests have low growth rates, and some understory tree seedlings seem to have low carbon gain. Very low photosynthetic rates are commonly reported from several species from different habit and life histories from the rain forest (Fetcher et al. 1983, Chazdon 1986, Denslow et al. 1990, McDonald and Strain 1991). Some simulated carbon gain studies have produced negative values for some understory tree seedlings species (Fetcher, Pers. Comm). Even some species can suffer photoinhibition due to lightflecks and no recovery can be observed during a given dark-time period (Le Gouallec et al. 1990).
On the other hand, nutrient response will depend on light availability (Oberbauer and Strain 1984, Riddoch et al. 1991). Non-pioneer species naturally growing in poor soils were less responsive to nutrient fertilization than pioneer species (Chapin 1980) and nutrient availability limits plant growth in landslides and similar early successional stages (Fetcher et al. 1996). Interactions among nutrients additions (like nitrogen and phosphorus) on landslides was also found by Fetcher et al. (1996) for the large herb Phytolacca icosandra. However, no nutrient effects were observed on some treelets and shrub species grown in light gaps, gap edges, and the understory (Denslow et al. 1990).
Bloom et al. (1985) suggested that plants minimize the cost of growth if the allocation is adjusted such that all resources are equally limiting to growth. For resources not directly limiting growth, Chapin (1991) mentioned that plant sink strength would be more important than resource availability in determining the rate of resource acquisition. This could be the reason why under limiting light, nutrient limitation is less severe than in habitats with increased light availability. The present work focuses on whether or not biomass allocation reflects limiting resources and whether or not the same resources limit different functions. This study investigates the importance of natural light conditions and nutrient availability (through nutrient addition) on growth and biomass allocation in the endemic herb, Begonia decandra (Begoniaceae) Pavón, from the wet subtropical forest of Puerto Rico. Despite the high diversity of tropical forest understories (Smith 1987, Gentry and Dodson 1987) and the importance of herbs in the gap-phase regeneration (Dirzo et al. 1992), experimental field research has been dominated by the study of trees, tree seedlings, and shrubs. However, some pioneer studies are available (Chazdon 1986, Sims and Pearcy 1992). Also, the importance of light levels in determining the response to fertilizer application has been stressed for regeneration stages in trees and other woody growth forms, but few studies have considered native understory herbs.
Materials and Methods
Begonia decandra is an understory herb from the eastern mountains of Puerto Rico. This species is found at elevations from 300 to 1000 m (Pers. obs.). However, I found natural populations growing in forest clearings and large gaps formed after the pass of Hurricane Hugo in Sept. 1989. The reproductive season generally begins in April and finishes in October, but sporadic blooming was observed in December 1991 and January 1992.
This study was conducted in the El Verde Field Station (EVFS), at the Luquillo Experimental Forest (LEF), in northeastern Puerto Rico (65° 49'W, 18° 20'N), which is located within the Subtropical Wet Forest life zone (Ewel and Whitmore 1973). Mean annual rainfall is 3920 mm. Sporadic dry periods occur between February and March (Brown et al. 1983), and can extend until April. Four light-gaps along the Sonadora Trail were chosen. These gaps were all almost flat areas within the western slope and were less than 200 m2 of ground open area. An understory area was also chosen adjacent to each gap. At the beginning of the experiment, measurements of the leaf area index (LAI, canopy leaf area divided by land area) were done with a LAI meter (Li-2000, LICOR, Nebraska, and U.S.A.), on May 2, 1991. LAI in three of the four gaps was 3.6, 2.62, and 2.14. Meanwhile values for the understory sites were 4.46, 4.81, 4.92, and 4.47. Some of the understory areas were heavily shaded due to the presence of several short palm trees (Prestoea acuminata).
Cuttings of several plants from different areas of the forest were collected and planted on one liter pots containing a commercial peatmoss mix for rooting and initially grown in a homogeneous environment (shade house with approximately 30% full sun radiation) constructed near EVFS. All pots were fertilized twice with a complete fertilizer (Peters Fertilizer Products, Fogelsville, PA, and USA). After one month, sixteen potted plants were randomly transferred to each forest environments (light gap and understory) in the eight sites, for a total of 128 plants. Half of the plants were randomly chosen for the nutrient addition treatment. These plants received fertilizer every two weeks during the first three months, and other application at the fifth month. Control plants did not receive fertilizer once they were transferred to the field. Both groups received a similar amount of water. At week four after transference to final sites, dead plants were replaced. Plant mortality was recorded four times during the course of the experiment. The harvest was done at the end of the seventh month (January 1992). During the harvest, pot media were carefully washed in order to obtain a maximum amount of roots. Leaves were cut, and the total leaf area per plant was obtained by cumulative measurements in a Leaf Area Meter (LICOR 3100, Nebraska, USA). Plant parts were dried in a convection oven at 60 C during 72 hrs. Below and aboveground biomass was obtained by adding up the dry weight of leaves, stems, flowers and fruits, and roots. The root to shoot ratio (RSR, root dry weight divided by aboveground dry weight), specific leaf mass (SLM, leaf dry weight divided by leaf area, g m-2), and leaf area ratio (LAR, total leaf area divided by the total plant biomass, in m2g-1) were calculated.
Initial and final plant mortality was analyzed with a two-by-two contingency test. Analysis of variance (ANOVA) was used for all other variables from the harvest. The ANOVA was used in a two-factor split-plot design laid off in blocks, considering each four forest microenvironment as the blocks (Plot effect), with the two light environments (light-gap and adjacent understory, Envi effect) as the whole plots per site and two subplots (fertilizer levels, Trt effect) per site (Ott, 1988). The PlotXEnvi mean square was used as error term for the Envi effect (Ott, 1988). Logarithm transformations were used to reduce variance and to adjust for non-normal distributions in the biomass and leaf area data.
Results
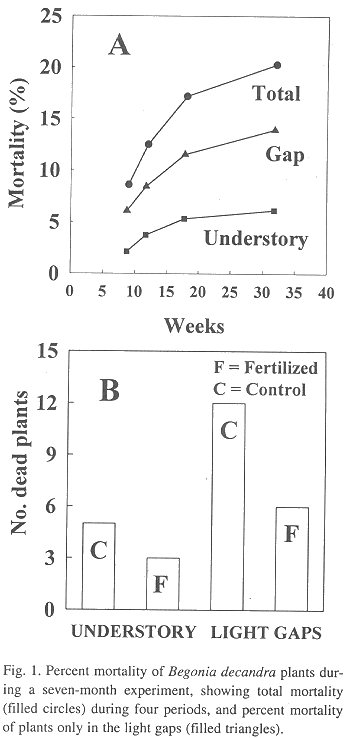
Plant mortality: Twenty-four plants were initially replaced by fourth week. Of these, 50% died in the added-nutrient treatment, but 67% plants died in the light gaps. Total plant mortality was 8.6% at week 9 and increased to 20.3% by week 32 (Fig. 1A, filled circles). With respect to number of total dead plants by week, approximately 70% of the dead plants were in the light gaps. This percentage remained constant through the entire experiment (Fig. 1B, filled triangles).
Analyses of all plant survival data classified by the treatment showed that survival is independent of the nutrient addition treatment (c2 = 2.37, P> 0.05), in spite of the fact that almost two-thirds (Fig. 1B) of the dead plants occurred in the control treatment. On the other hand, plant survival was associated with the light environment, indicating that twice the number of plants disappeared in the light gaps (18 plants) in comparison with the number of plants that died in the understory (8 plants, Fig. 1B, c2 = 3.91, P<0.05). However, given the small number of dead plants between treatments, this test was considered not significant. In addition, the two-by-two contingency test performed on the combined data (Fig. 1B) indicated that mortality in the nutrient treatments were independent of mortality in the two forest environments (c2 = 0.058, P> 0.05).
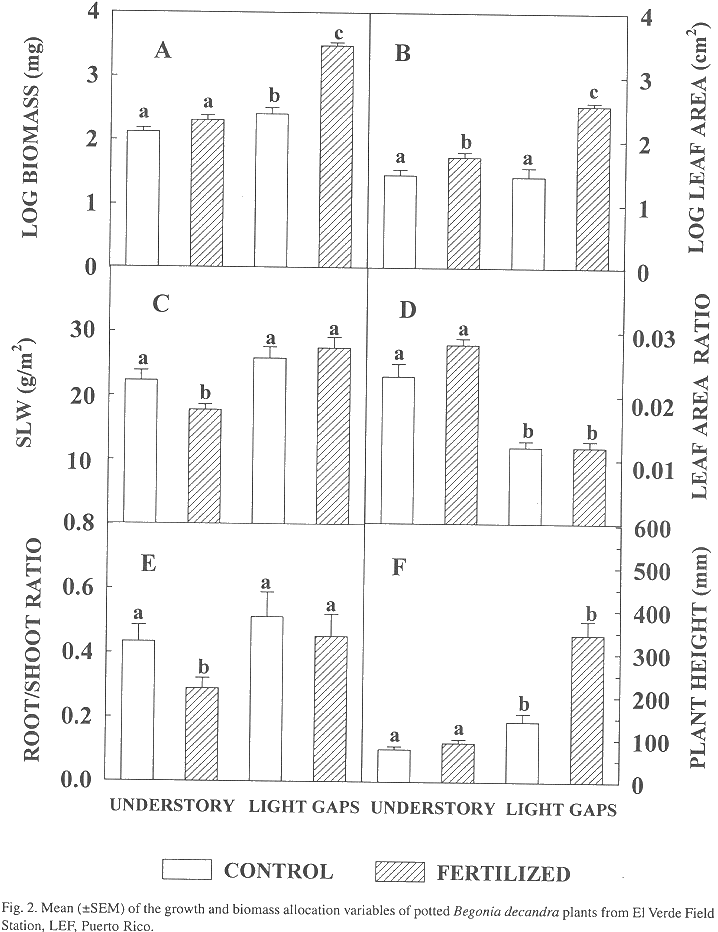
Growth and biomass allocation: At harvest, total height of Begonia plants was greater in the light-gap environments than in the understory environments (Fig. 2, Table 1). The light-gap environment had a positive significant effect on plant height, biomass, and SLM (Table 1, Fig. 2). There was a significant effect of plot on the leaf area, RSR, and SLM. The interaction between light environment with plot was significant for the leaf area and RSR. In general, the variables related to absolute growth (height, biomass and leaf area) were strongly favored on sites with higher resources.
There was a significant effect of nutrient addition in the plant height, biomass, leaf area, and SLM (Table 1, Fig. 2). The interaction between treatment effect with environment was significant in all these four variables. The nutrient addition had positive effects on plant height, leaf area, and biomass of plants growing in the light gaps, with no evidence of significant effect of the SLM, LAR and RSR (Fig. 2). Nutrient addition resulted in lower mean values of SLM and RSR in the understory sites (Fig. 2). In general, the variables related to allocation (SLM and RSR) showed a tendency to change significantly to the addition of nutrients when growing under low light conditions.
TABLE 1
Probabilities of the F ratios and the coefficient of determination for the split plot ANOVA.
| | |||||||
| Parameters | | | | | | | |
| Plant Height (cm) | | | | | | | |
| Biomass (Log) | | | | | | | |
| Leaf area (Log) | | | | | | | |
| RSR | | | | | | | |
| LAR | | | | | | | |
| SLM | | | | | | | |
Abbreviations: Envi, forest environment; Trt, fertilization treatment; R2, Coefficient of determination; P, probability; ns, not significant at the 0.05 level; *, P< 0.05; **, P< 0.01. RSR, root to shoot ratio; LAR, leaf area ratio; SLM, specific leaf mass.
Discussion
Most variables analyzed in this study show that growth of Begonia decandra is greatly enhanced when situated under higher light conditions like the ones provided by the forest light-gaps, when compared with its development in understory conditions. This response has been found in many plant species in different forests (Oberbauer and Strain, 1985; Popma and Bongers 1991; Fetcher et al. 1994). Plant mortality, however not strongly significant, it tended to be higher in the light gap environments. Sudden changes from low-light to high-light environments could be deleterious and commonly produce stress responses such as strong leaf loss, photobleaching, and mortality (Fetcher et al. 1983, 1987; Strauss-Debenedetti and Bazzaz, 1991. Even sequences of longer sunflecks induce photoinhibition which is cumulative and resemble prolonged exposure to high light regimes in the understory rain forest herb Elatostema repens (Le Gouallec et al. 1990). However, forest light gaps provide a set of microenvironmental conditions in addition to higher light, such us greater vapor pressure deficit and higher temperature (Fetcher et al. 1985), which can result in greater transpirational demand. It is likely that B. decandra mortality in the light gaps would be associated with all these drastic changes. A little higher mortality and greater growth in the light gaps could be also a result of root constriction and other pot effects. The possible effect of pot size would predict that mortality rate would increase with time, as plants grow. However, plant mortality in gaps was proportionally constant with the mortality in the understory (Fig. 1A). So, this species seems rather light intolerant. However, the majority of plants overcame the transition period and stressing conditions of the new lighter condition, and produced greater growth.
As in this study, many other studies on growth and biomass allocation have found a tendency to increase biomass, leaf area, SLM, RSR and to decrease LAR in response to increased light availability (Fetcher et al. 1983; Kamaluddin and Grace, 1993; Fetcher et al. 1994). Increases in LAR has been associated with reduced red/far red ratio of understory environments (Kwesiga and Grace, 1986), but this factor was not considered in this study. In general, the effect of the light environment, the nutrient addition, and the light by nutrient interaction emphasize the dependency of the plant performance from light in terms of total growth (plant height, biomass and leaf area), where the highest growth is expected in the more resource-rich environment (higher light and nutrients). Similar results where obtained by Riddoch et al. (1991).
In spite of great differences in biomass and leaf area between treatments, differences of SLM and RSR were smaller, even absent in LAR, especially on the plants grown in light gaps. It would appear that increased nutrient availability had less impact on changes in allocation patterns when the plants were growing with greater light availability. This difference in the importance of a resource over another emphasizes the relevance of light-gap conditions in the resource allocation patterns displayed by B. decandra for the maintenance of balanced growth. This response has been reported for other tropical plants. For example, increases in the parameters of the light response curve of treatments under nutrient supply have had smaller influences than those due to light growing conditions (Riddoch et al. 1991). Studies with Alocasia (Sims and Pearcy 1992), acclimation to high light seems determined by the leaf area production and photosynthetic capacity, mediated by leaf thickness and construction costs. So, Begonia acclimation capacity to these forest gaps does not seems strongly determined by the effects of nutrient addition.
On the other hand, the tendency to produce greater differences between nutrient treatments in RSR and SLM of plants grown under shade enhances the importance of light as the main factor influencing absolute growth. But this also suggests a greater resource allocation response to nutrient availability in the light environment where the species is more common, the shaded understory. Then, it seems that plants always maintain a balanced growth depending on the resource availability, its acclimation ability, and the original light growing conditions. For this understory plant, when light conditions are low, changes in other resources alter biomass allocation patterns with relatively greater intensity.
The three parameters that describe absolute growth (height, biomass and leaf area) showed a significant interaction between light environment and plot, which illustrates the importance of particular site differences in light availability on the total growth in this species, especially taking into consideration the differences in the LAI among the chosen light gaps.
In conclusion, while B. decandra plants grow and survive in understory environments, its productivity was greatly modified when grown under increased light environments, as the ones produced by tree-fall gaps. It was evident that total growth was significantly affected by the addition of nutrients, and the interaction effect with the environment was strong. However, the biomass allocation variables changed more due to added nutrients when plants were grown under limited light conditions. In addition, light differences due to site can cause significant changes in the parameters related to the total growth. These last two aspects do not support a previous hypothesis (Bloom et al. 1985), because allocation was not adjusted in all cases, not all resources are equally limiting growth, so the cost of growth is higher under limited light (Bloom et al. 1985). Given the changes in allocation patterns produced by the nutrient treatments under limited light, this study do not support the idea that sink strength is more important than resource availability in determining the rate of resource acquisition, as suggested by Chapin (1991).
Resumen
Se estudia el efecto de dos regímenes de luz natural (claros del bosque y el sotobosque) y su interacción con la disponibilidad de nutrimentos sobre el crecimiento y la repartición de biomasa de Begonia decandra, una hierba del sotobosque en el Bosque Experimental de Luquillo, Puerto Rico. Dieciseis plantas en macetas obtenidas por estacas se asignaron aleatoriamente a cada uno de los ocho sitios del bosque (cuatro en claros y cuatro en el sotobosque), para un total de 128 plantas. Se aplicó fertilizante completo a la mitad de las plantas de cada sitio. Después de siete meses en los ambientes estudiados, la adición de nutrimentos afectó positivamente el crecimiento total y produjo algunos cambios en los patrones de repartición de biomasa. La respuesta general en las variables estudiadas fue similar a las respuestas obtenidas en plántulas y brinzales de árboles de otras regiones tropicales. La biomasa y el área foliar fueron muy sensibles al incremento del recurso principal (luz). La fertilización afectó menos los patrones de asignación de biomasa (relación raíz/vástago, masa foliar específica y cociente de área foliar) cuando las plantas están en los claros, y estos cambios fueron más pronunciados cuando el recurso principal es limitante (en el sotobosque). Las diferencias lumínicas debidas al sitio pueden causar diferencias importantes en las variables relacionadas al crecimiento total. La mortalidad de plantas en los tratamientos con nutrientes fue independiente de la mortalidad en los tratamientos lumínicos. Algunas hipótesis sobre adquisición de recursos y crecimiento vegetal no son sustentadas por este estudio.
Acknowledgements
I thank J. D. Ackerman for suggesting to me the species for this work. This study was funded by the Intercampus Ph. D. Program and the Biology Department of the University of Puerto Rico. Shi Yun Wen, E. Meléndez and two anonymous reviewers made significant improvements to previous versions of this manuscript.
References
Bloom, A.J., F.S. Chapin III, & H.A. Mooney. 1985. Resource limitation in plants- an economic analog. Annu. Rev. Ecol. Syst. 16: 363-392. [ Links ]
Brown, S., A.L. Lugo, S. Silander & L. Liegel. 1983. Research History and opportunities in the Luquillo Experimental Forest. Gen. Tech. Rep. S-44. Institute of Trop. Forestry Publ. Southern For. Exp. Sta. USDA. Louisiana. [ Links ]
Chapin, F.S. III. 1980. The mineral nutrition of wild plants. Annu. Rev. Ecol. & Syst. 11: 261-285. [ Links ]
Chapin, F.S., III. 1991. Integrated responses of plants to stress. BioScience 41: 29-36. [ Links ]
Chazdon, R.L. 1986. Light variation and carbon gain in rain forest understory palms. J. Ecol. 74: 995-1012. [ Links ]
Denslow, J.S., J.C. Schultz, P.M. Vitousek & B.R. Strain. 1990. Growth responses of tropical shrubs to treefall gap environments. Ecology 71: 165-179. [ Links ]
Dirzo, R., Horvitz, C.C., Quevedo, H., & López, M.A. 1992. The effects of gap size and age on the understory herb community of a tropical Mexican rain forest. J Ecol. 80: 809-822. [ Links ]
Ewel, J.J. & J.L. Whitmore. 1973. The ecological life zones of Puerto Rico and the Virgin Islands. U.S. For. Serv. Pap. ITF-18. Institute of Tropical Forestry, Río Piedras, Puerto Rico. [ Links ]
Fetcher, N, B. R. Strain & S. F. Oberbauer. 1983. Effects of light regime on the growth, leaf morphology, and water relations of seedlings of two species of tropical trees. Oecologia 38: 314-319. [ Links ]
Fetcher, N., S. F. Oberbauer & B. R. Strain. 1985. Vegetation effects on microclimate in a lowland tropical forest in Costa Rica. Int. J. Meteor. 29: 145-155. [ Links ]
Fetcher, N., S. Oberbauer, G. Rojas & B.R. Strain. 1987. Efectos del regimen de luz sobre la fotosíntesis y el crecimiento en plántulas de árboles de un bosque lluvioso tropical de Costa Rica. Rev. Biol. Trop. 35(suppl. 1): 97-110. [ Links ]
Fetcher, N. S.F. Oberbauer & R.L. Chazdon. 1994. Physiological ecology of plants, p. 128-141. In McDade, L.A., K.S. Bawa, H.A. Hespenheide & G.S. Hartshorn (eds.) La Selva: Ecology and natural history of a neotropical forest. The University of Chicago Press, Chicago. [ Links ]
Fetcher, Ned., B.R. Haines, R. A. Cordero, D.J. Lodge, L.R. Walker, D.S. Fernández & W.T. Lawrence. 1996. Responses of tropical plants to mineral nutrients and light on a landslide in Puerto Rico. J. Ecol. 84: 331-341 [ Links ]
Gentry, A.W. & Dodson, C.D. 1987. Contribution of non-tree species to species richness of tropical rain forest. Biotropica 19, 149-156. [ Links ]
Kamaluddin, M. & J. Grace. 1993. Growth and photosynthesis of tropical tree seedlings (Bischofia javanica Blume) as influenced by a change in light availability. Tree Physiol. 13: 189-201. [ Links ]
Kwesiga, F. & J. Grace. 1986. The role of the red/far red ratio in the response of tropical tree seedlings to shade. Ann. Bot. 57: 283-290. [ Links ]
Le Gouallec, J. L., G. Cornic & P. Blanc. 1990. Relation between sunfleck sequences and photoinhibition of photosynthesis in a tropical understory rain forest herb. Am. J. Bot. 77: 999-1006 [ Links ]
McDonald, E. P. & B.R. Strain. 1991. Photosynthetic response of four tropical species of Miconia to sustained increases in irradiance. Bull Ecol. Soc. Am. 72: (suppl.): 186. [ Links ]
Oberbauer, S. F. & B. R. Strain. 1984. Photosynthesis and successional status of Costa Rican rain forest trees. Photosynth. Res. 5: 227-232 [ Links ]
Oberbauer, S. F. & B. R. Strain. 1985. Effect of light regime on the growth and physiology of Pentaclethra macroloba (Wild.) Kuntze, in Costa Rica. J. Trop. Ecol. 1: 303-320. [ Links ]
Ott, L. 1988. An introduction to statistical methods and data analysis. PWS-Kent Publishers Co. Boston. 835 p. [ Links ]
Popma, J. & Bongers. 1991. Acclimation of seedlings of three Mexican rain forest tree species to a change in light availability. J Trop Ecol 7: 85-97. [ Links ]
Riddoch, I., T. Lehto & J. Grace. 1991. Photosynthesis of tropical tree seedlings in relation to light and nutrient supply. New Phytol. 119: 137-147. [ Links ]
Sims, D. A. & R. W. Pearcy. 1992. Response of leaf anatomy and photosynthetic capacity in Alocasia macrorrhiza (Araceae) to a transfer from low to high light. Amer. J Bot. 79: 449-455. [ Links ]
Smith, A. P. 1987. Respuestas de hierbas del sotobosque tropical a claros ocasionados por la caída de árboles. Rev. Biol. Trop. 28(Suppl. 1): 111-118. [ Links ]
Strauss-Debenedetti, S. & F.A. Bazzaz. 1991. Plasticity and acclimation to light in tropical Moraceae of different successional status. Oecologia 87: 377-387. [ Links ]
1Dept. of Biology, University of Puerto Rico, Río Piedras, PR 00931-3360 USA
Present address: Smithsonian Tropical Research Institute, P. O. Box 2072, Balboa, Panama. E-mail: corderor@bci.si.edu. Fax: (507) 272-3065.












 uBio
uBio