Introduction
Seed dispersal is one of the most important interactions in tropical forests (Howe & Smallwood, 1982; Willson & Traveset, 2000). It is fundamental for the subsistence of plant communities over time (Cain et al., 2000; Chazdon, 2003; Stevenson, 2011), since frugivores can modify demographic processes, population dynamics, potential recruitment area, and density-dependent processes (Connell et al., 1984; Janzen, 1971; Nathan & Muller-Landau, 2000; Willson, 1993). Several organisms perform seed dispersal services in different ecosystems, where birds and mammals have been suggested as the main ones dispersing seeds of many tropical plant species (Howe, 1990).
Bats have been considered the main nocturnal seed dispersers (Fleming & Heithaus, 1981; Mello et al., 2011). Different studies have cataloged bats as efficient seed dispersers from a quantitative point of view, finding that they move a high number of seeds by consuming large amounts of fruit, even more than birds (Brosset et al., 1996; Duncan & Chapman, 1999; Medellin & Gaona, 1999). Bats are also effective seed dispersers, with high germination percentages after seeds pass throughout their digestive system (Fleming, 1988; Vasquez-Yanes & Orozco-Segovia, 1986).
Some authors highlight bats as important seed dispersers because when defecating in flight they are more likely to deposit seeds in forest clearings and open areas than birds and primates, playing a very important role in forest regeneration (Korine & Kalko, 2005; Thies & Kalko, 2004; Thomas et al., 1988). For other nocturnal mammals such as opossums (Didelphidae), raccoons and mountain dogs (Procyonidae), and rodents (Rodentia) efficient seed dispersal has also been observed (Fleming & Williams, 1990; Howe, 1990; Jansen et al. 2012).
In contrast, diurnal birds have been considered the main dispersers in a variety of habitats (Howe, 1977; Wang & Smith, 2002; Wenny et al., 2016). Birds have been described as efficient seed dispersers due to their wide home ranges, flight capacity, broad frugivorous diet, and the likelihood of recruitment (Herrera & Jordano, 1981; Levey et al., 2005; Stiles, 1980). Due to the great morphological, behavioural, and ecological diversity of frugivorous birds, it has been found that they are also important in the restoration of forests and especially increasing the connectivity of patches isolated as a consequence of forest fragmentation, in addition to promoting plant diversity (Carlo & Morales, 2016; Emer et al., 2018). As a particular case in neotropical forests is a nocturnal frugivorous bird known as oilbird (Steatornis caripensis), which presents a diverse diet of fruits and seeds, and a great capacity to disperse a high number of seeds (Stevenson et al., 2017).
On the other hand, in tropical zones, it has been shown that diurnal primates play disproportionate ecological roles as seed dispersers (Link & Di Fiore, 2006). Because of their high abundance and large size relative to other frugivores in many tropical forests (Bravo, 2012; Campbell et al., 2016), their large biomass, and their ability to ingest large seeds, primates are also considered efficient seed dispersers (Chapman & Dunham, 2018; Stevenson et al., 2005). Additionally, they disperse high amounts of seeds over long distances, are effective seed dispersers, and their seed dispersal pattern shows a high correlation with seedling recruitment (Gelmi-Candusso et al., 2019; Stevenson et al., 2002; Zárate et al., 2019).
The purpose of this study is to compare daytime and nighttime seed dispersal, as an approximation to seed dispersal roles of diurnal and nocturnal arboreal and flying frugivores in a secondary and primary sub-Andean forest of Colombia. We expect to find differences in the amount, biomass and species of seeds dispersed between day and night.
Materials and methods
Study area: The study was conducted in the Cueva de Los Guácharos National Natural Park (PNNCG) located in the department of Huila and the Southwestern part of the department of Caquetá (Colombia). The predominant habitat is a sub-Andean forest, both primary and secondary, the latter being a forest with a growth close to 40 years (Prada & Stevenson, 2016). The study area is on average at 2 000 m.a.s.l. with a mean temperature of 18.8 °C. The annual precipitation is 2 284 mm and the rainy season presents a bimodal pattern with a first peak between March and June, and a second peak between September and October. Species richness of different organism groups has been partially registered for PNNCG. Prada and Stevenson (2016) report 458 species/morphospecies of trees, about 200 bird species (Stevenson et al., 2022), 10 species of medium and large mammals (Gast & Stevenson, 2021), 11 species of small nonflying mammals (Bautista-Plazas et al., 2017), 5 species of primates (Vargas et al., 2014) and 25 species of bats (Walteros-Vergara, 2018).
Seed dispersal: Hanging traps of 0.56 m² were used to capture the dispersed seeds, the porosity of the traps was < 1 mm to ensure that small seed species were represented in the study. The material was cloth and they were tied to four nearby trees, following the recommendations of Stevenson & Vargas (2008). A total of 60 traps were arranged in four 1 km long linear transects, two of these transects were placed in the secondary forest and two in the primary forest, each with 15 hanging traps. The location of each trap within the transect was determined by three random numbers: 1. The space between adjacent traps (between 15 and 20 m), 2. The side of the trails (right or left), and 3. The distance to the trails (between 1 and 10 m).
Traps of each transect were checked before sunrise (5:00) and before sunset (17:00) for 10 continuous days every two months (February, April, June, August, October, December) throughout 2017. Once the contents were collected, the seeds were identified at the species level when possible. This material was dried in a portable oven at an average temperature of 60 °C to achieve constant dry weight. Then, we counted the number of dispersed seeds and estimated the biomass of dispersed seeds (kg/ha) (Stevenson, 2004). Seeds were classified as dispersed when they were associated with dung and all the adult trees, lianas, and shrubs within a 10 m radius of each hanging trap were identified to discern whether the seeds deposited in the trap were dispersed or came from nearby parents (Stevenson, 2007). Besides, it was corroborated that they were dispersed seeds since there were no immature fruits, whole fruits, or flowers of the same species in each trap.
Statistical analysis: A linear mixed model was used to evaluate the fixed effects as time (day/night) and forest type (primary/secondary), and month as a random effect (February, April, June, August, October, December), on the logarithm of the number of dispersed seeds and the logarithm of dispersed seeds biomass (kg/ha). The linear mixed model was carried out using ''lme4'' and ''lmerTest'' packages from software R 4.2.3 (R Core Team, 2023). We also estimated random effects for each month and their interval estimate using ''merTools'' package. We calculate similarity between seed species dispersed in the day and seeds dispersed in the night with the Jaccard similarity index using ''Vegan'' package.
Results
A total of 93 species of seeds were identified, 65 species were dispersed during the day and 56 during the night. We found that around half of the species dispersed in the day were not shared with the species dispersed in the night (Jaccard similarity index = 0.54). Twenty-eight species were exclusively dispersed during the night and 37 during the day.
The plant species with the highest seed biomass dispersed during the day were Alchornea grandis, Tapirira guianensis subandina, Chamaedorea linearis, Guettarda crispiflora, and Rollinia dolichopetala.
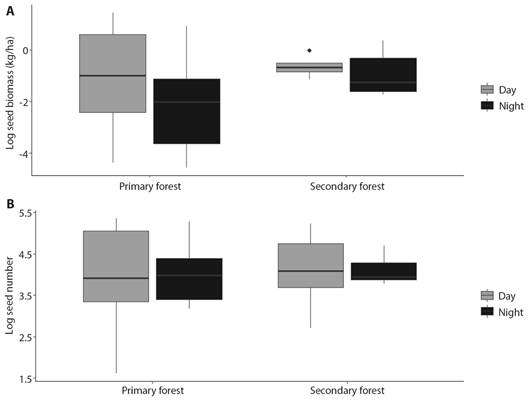
Fig. 1 A. Biomass of seeds dispersed (kg/ha) day and night at secondary and primary forest in PNNCG. B. The number of seeds dispersed day and night at secondary and primary forest in PNNCG.
On the other hand, the plant species with the highest seed biomass dispersed at night were Tapirira g. subandina, Sapium stylare, Hyeronima huilensis, Rollinia dolichopetala and Anthurium multinervium.
The plant species with the highest number of seeds dispersed during the day were Ficus sp.1, Cecropia telenitida, Solanum sp.1, Morus insignis, and Cecropia angustifolia. In contrast, at night the highest number of dispersed seeds were recorded for Ficus sp.1, Cecropia telenitida, Anthurium multinervium, Solanum sp.1, and Hieronyma andina.
We recorded a total of 1 874 effectively dispersed seeds, of which 1 046 (55.8 %) were collected during the day and 828 (44.2 %) at night. The total dispersed seed biomass was higher in the day with 10.96 kg/ha, while for night it was 6.39 kg/ha; however, the variation was large and no significant differences were found (T = -1.25, P = 0.22, Fig. 1A). Similarly, no significant differences were found between the number of seeds dispersed during the day and night (T = 0.18, P = 0.85, Fig. 1B).
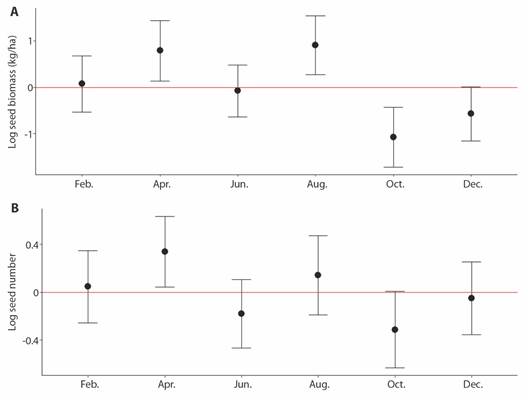
Fig. 2 A. Biomass of seeds dispersed (kg/ha) through the year at PNNCG. B. The number of seeds dispersed through the year at PNNCG.
In the primary forest, a total of 995 seeds were dispersed (551 during the day and 444 at night) and 879 (495 during the day and 384 at night) in the secondary forest, no significant differences were found in the number of seeds between forest types (T = 0.41, P = 0.68, Fig. 1B). Additionally, we did not observe a significant difference between the primary and secondary forests in the biomass of dispersed seeds (T = 1.58, P = 0.13, Fig. 1A), a total of 10.79 kg/ha of seeds was dispersed in the primary forest and 6.57 kg/ha in the secondary forest. No significant differences were found in the number or biomass (kg/ha) of seeds dispersed between the months sampled. However, April and August recorded the highest number and biomass of dispersed seeds, while in October and December the lowest seed dispersion (Fig. 2).
Discussion
Both, birds and bats, have been widely regarded as the main seed dispersers of day and night seeds respectively, in terms of the number of seeds transported (Fleming & Heiathus, 1981; Janson, 1983; Levey et al., 2002; Mello et al., 2015; Wenny & Levey, 1998). In this study, we did not observe marked differences in the number of seeds dispersed during the day and night, nor in the number of plant species dispersed in these two periods. Our results agree with what was found for trees isolated within grasslands by Galindo-González et al. (2000), however, for other tropical forests, a greater dispersion of seeds by one of the two large groups (birds or bats) has been reported (Gonzales et al., 2009; Ingle, 2003; Jacomassa & Pizo, 2010;).
We did not find a greater nocturnal seed dispersal for any of the studied forests (primary and secondary), although we observed a slightly higher seed biomass at night in secondary forests than in primary forests. This contrasts with what was found by other authors where bats are more important in successional forest states where they disperse significantly more seeds and are important for the natural regeneration of ecosystems (Duncan & Chapman, 1999; Medellin & Gaona, 1999). This can be explained by the fact that secondary forests in the study area are relatively old (ca. 40 years in recovery).
Similarly, it has been suggested that birds have greater importance in terms of seed dispersal within mature forests than in disturbed forest in recovery and with fragmentation (Ingle, 2003; Whittaker & Jones, 1994). However other studies support that seed dispersal by birds is crucial in forest regeneration for disturbed habitats (Carlo & Morales, 2016; Saavedra et al., 2014). In our case, although there are no notable differences in diurnal seed dispersal between primary and secondary forests, our results show a greater value in both the number of seeds and the biomass of seeds dispersed by diurnal animals for the secondary forest.
For our study forest, there was high variability in the dispersion of seeds at day and night, being slightly higher during the day both in biomass and number of dispersed seeds. The slightly higher biomass and seed count during the day is expected since not only bird droppings but also primate droppings were found in seed traps collected at day-time In the PNNCG study site, it has been reported that the most abundant primates in the area are the woolly monkeys (Lagothrix lagothricha) who disperse a large number of seeds, with an average of 11 000 seeds km² day-¹ (Ramírez et al., 2014). This explains the high dispersion of large seeds such as Tapirira g. subandina, Sapium stylare, and Rollinia dolichopetala which are known to be heavily consumed and dispersed by woolly monkeys (Ramírez et al., 2014). Additionally, in the area there are other species of primates such as Sapajus apella, Alouatta seniculus and Saimiri sciurus, which could also disperse large seeds (Chapman, 1995), but occur at very low density (Vargas et al., 2014).
Within the seeds dispersed at night, we found a high number of seeds from Tapirira g. subandina which is the most important plant for the population of woolly monkeys and has not been reported in the diet of bats. Therefore, we believe that this species and other plants preferred by monkeys (e.g. Rollinia dolichopetela) are dispersed during nighttime by primates in their sleeping trees. These nocturnal depositions by primates are important and should be considered for nocturnal dispersal as they can comprise up to 19 % of the total seeds dispersed during the day by primates (Stevenson, 2007). We cannot rule out that these seeds were dispersed by other nocturnal organisms such as nocturnal monkeys (Aotus sp), Potos flavus, or marsupials, but presumably, the effect of the wooly monkeys could be greater, given their high abundance (ca. 20 individuals/km2) (Vargas et al. 2014). It is also important to bear in mind that the study area has a large oilbird colony (Steatornis caripensis). This species could also be responsible for nocturnal seed dispersal of species such as Chamaedorea linearis, Hedyosmum cuatrecazannum and Nectandra sp. which are part of the diet of this nocturnal bird (Stevenson et al., 2017). Nevertheless, nocturnal seeds dispersal from species such as Tapirira g. subandina, Rollinia dolichopetala and Hieronyma huilensis, probably come from diurnal frugivores, because of their size and not being registered in the oilbird's diet.
Our observations indicated that the trends in both the number and amount of biomass of seeds dispersed at night and daytime generally kept the same trend overtime with April being the month of greatest dispersal and October the one with the least dispersal. Seed dispersal at this location seems to be affected mainly by changes in fruit production throughout the year. In fact, the months with higher fruit production also showed a peak in the number of dispersed seeds (Bautista, unpublished). Additionally, when there is greater production of fruits, more visits of fruit eaters are expected. So, it seems that it could be important in future studies to evaluate productivity as predictor of the amount of seed dispersion, as has been observed in other studies (Albrecht et al. 2012; Hampe, 2008).
Knowing the dispersion of seeds in forests with different degrees of disturbance is of vital importance to understand the processes of natural regeneration (Gorchov et al., 1993; Uriarte & Chazdon, 2016). It is also essential to estimate the contribution of different organisms to the seed rain of particular species within the plant community (Howe, 1990). From the results found in this study, we can say that for the primary and the old secondary sub-Andean forests of the PNNCG, share many frugivores such as primates and birds (Vargas et al., 2014), which may explain the lack of differences in diurnal and nocturnal dispersal.
Our main conclusion was diurnal and nocturnal dispersal but that is not saying much about seed dispersal by birds and primates vs. bats, and that in natural tropical forests the role of primates and nocturnal birds might be substantial, where they reach high densities and are not subject to hunting (Howe, 1990; Stevenson, 2011; Stevenson et al., 2017; Wright et al., 2000). For this reason, it is imperative to generate conservation strategies to protect the different species of seed-dispersing organisms, since the persistence of multiple species and ecosystems depends on them (Chazdon & Guariguata, 2016; Kozlowski, 2002). By preserving these organisms, damages to the plant community such as deforestation, fragmentation, and loss of species can be ameliorated and remedied (Bacles et al., 2006; Cramer et al., 2007; McConkey et al., 2012; Ozinga et al., 2009), because frugivores help in the processes of regeneration and restoration of degraded forests (Parrotta et al., 1997).
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgments section. A signed document has been filed in the journal archives.












 uBio
uBio 


